| Issue |
J Oral Med Oral Surg
Volume 24, Number 1, January 2018
|
|
|---|---|---|
| Page(s) | 11 - 21 | |
| Section | Revue de la littérature / Literature review | |
| DOI | https://doi.org/10.1051/mbcb/2017030 | |
| Published online | 25 May 2018 | |
Literature Review
Therapeutic uses and efficacy of botulinum toxin in orofacial medicine
1
Resident at Paris Hospitals,
Paris, France
2
Maxillofacial Surgery Service, Hôpital Pitié Salpêtrière,
Paris, France
3
Maxillofacial Surgery Service, Georges-Pompidou European Hospital,
Paris, France
4
Dental Surgery Service, Bretonneau Hospital,
Paris, France
5
Faculty of Dental Surgery, Paris Descartes University,
Montrouge, France
* Correspondence: bayet.kinz@gmail.com
Received:
13
November
2016
Accepted:
10
September
2017
Introduction: Botulinum toxin, primarily known for its use in cosmetic surgery, is also used for therapeutic purposes in many medical fields. It works as a muscle relaxant and inhibits glandular secretions. In the orofacial sphere, the use of this toxin is proposed in particular for disabling myofascial pain and aberrant salivary disorders. Methodology: A critical analysis of the literature, based on PUBMED data, concerning the orofacial indications of botulinum toxin was carried out. Results: The literature is abundant regarding the therapeutic interest of this toxin for several oral pathologies, but scientific merits vary markedly from one indication to another. Discussion: The musculorelaxant and antisecretory action of this toxin appears to be demonstrated in the case of bruxism, limitation of the mouth opening and in hypersialorrhea. On the other hand, its medical benefit is still not supported by scientific evidence for masseter hypertrophy, tonicity of the levator labii superioris muscles, sialocele fistulae and Frey's syndrome. Conclusion: Additional high-level studies, unbiased, randomized controlled trials, are required to eliminate the uncertainties that persist about the clinical impact of botulinum toxin and to justify the development of recommendations for good practice valid and credible.
Key words: botulinum toxin / myofascial pain syndrome / sialorrhea
© The authors, 2018
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Botulinum toxin is a complex protein produced by gram-positive anaerobic bacteria of the Clostridium genus. To date, seven serotypes of the toxin, i.e., A–G, have been identified according to their different antigenic properties, but only A and B serotypes are available commercially. The serotypes are 300–900 kDa in size, and each is composed of a neuroactive entity [1] called botulinum neurotoxin (BoNT), which binds with a nontoxic protein that helps to stabilize the complex protein [2].
Botulinum toxin is one of the most dangerous poisons known to man. It is 40 million times more toxic than cyanide. This powerful toxin has a potent biological effect: it is able to block the release of vesicular acetylcholine and consequently inhibits the muscles fibers activation. This inhibition is however transient, which makes it clinically relevant. It is, in fact, the reversible ad integrum action of the toxin, which makes it safer to use for therapeutic purposes. Preventing acetylcholine release affects both the neuromuscular junctions and other types of synapses. Depending on the target tissue, the toxin may block cholinergic innervation of skeletal muscles, but it also may block cholinergic innervation of the exocrine glands [2–4]. Since the 1980s, many medical indications of the toxin have emerged. The principle is to utilize the paralytic properties of the toxin so as to eliminate excessive or even painful muscle contractions. Although the use of the toxin is widespread in cosmetic surgery, it is also used in many other specialties: in gastroenterology, it is used in cases of dysphagia, achalasia, and esophageal motor disorders; in urology, it is used in cases of overactive bladder and vescicosphincteric disorders; in otolaryngology, it is used in cases of hemifacial spasms, spasmodic dystonia, and velar myoclonus; in ophthalmology, it is used in cases of blepharospasm; and in dermatology, it is used to treat conditions such as hyperhidrosis.
Orofacial uses have been under explored, despite a definite therapeutic potential. The use of botulinum toxin, in fact, offers an attractive therapeutic alternative in managing myofascial pains such as bruxism and masseter hypertrophy. The toxin may be used in the treatment of hypertonicity of the superior labial muscles, mouth opening limitation (MOL), aberrant salivary disorders, sialo-oral fistulas, and Frey syndrome. Far from being systematically prescribed for the latter indications, the use of this toxin is generally reserved for clinical situations that are refractory to first-line treatments. In some cases, its use may nevertheless be a first-line treatment. The therapeutic benefits are significant: the injection of botulinum toxin is a simple, minimally invasive practice, and this treatment makes it possible to significantly improve the patient's living conditions. The aim of this study is to take a look at the current literature data on the orofacial indications of botulinum toxin.
Materials and method
Studies and reviews of the literature from 2006 up to and including 2016 concerning the treatment of myofascial pains of the masticatory muscles and aberrant salivary disorders have been selected from the PUBMED database. The key words used were as follows: “botulinum toxin,” “myofascial pain,” “bruxism,” “masseter hypertrophy,” “jaw constriction,” “gummy smile,” “drooling,” “sialorrhea,” “fistula,” and “Frey's syndrome.” Low-statistical-power, poorly conducted, noncomparative, non-randomized studies or studies that did not address the subject matter were not selected given their low scientific merit, according to HAS (Haute Autorité de Santé) [High Health Authority] recommendation guidelines. For the purpose of this article, the results have been classified by pathology.
Results
Muscular pathologies
Myofascial pathologies combine two clinical features: myalgia of the mandibular elevator muscles and muscular hypertonicity of the superior labial muscles.
Bruxism
Sixty-two articles and journals were found. After the reading of all abstract, only studies with a high scientific merit were selected. In total, eight studies, focusing on the treatment of musculoskeletal pain in patients with bruxism were selected [5–12] These studies all had a high level of evidence according to HAS. The studies are summarized in Table I. The common purpose of these studies was to compare the effect of botulinum toxin and a saline placebo solution on bruxism-related myofascial pain. A positive outcome was found in the majority of studies; four out of eight studies [5,6,9,10] reported a significant decrease in pain, which led to the conclusion that botulinum toxin was effective in patients with bruxism.
Critical analysis of the literature on the efficacy of botulinum toxin in the management of bruxism.
Masseter hypertrophy
A total of 33 articles concerning the treatment of masseter hypertrophy were selected and 32 were excluded: Eleven did not evaluate the effiency of botulinum toxin, 21 were part of a case series or were studies of low scientific merit (grade C). Only a systematic review of the literature published by Cochrane in 2013 [13] was finally retained. This review revealed the lack of evidence regarding the efficacy of botulinum toxin in the management of masseter hypertrophy while emphasizing the existence of promising results.
MOL
The analysis of the literature revealed 21 articles: Twelve articles did not deal with subject at hand (either the pathology or the botulism toxin treatment), six were regarding MOL associated with systemic diseases (the treatment of these MOL cases were via the treatment of systemic diseases), and three were clinical case reports of low scientific merit. Thus, a total of five items of medium to strong scientific merit [14–18] were included in our analysis; the details of these studies are presented in Table II. A significant clinical improvement was found in the three studies of high scientific merit: Fietzek [14], Guarda-Nardini [15], and Kurtoglu [16]. Denglehem's prospective study [17] emphasized improvement in the patient's living conditions. It showed the painkilling effect of botulinum toxin through muscle relaxation, which decreases the pressure on Temporomandibular joint (TMJ), inflammation, and therefore pain.
Critical analysis of the literature on the efficacy of botulinum toxin in the management of mouth opening limitation.
Hypertonicity of the levator labii superioris muscles
Over the past 10 years, 18 articles analyzed the efficacy of botulinum toxin in the management of gingival smiles. Seventeen were clinical case reports of low scientific merit. Sixteen of them included less than five subjects, who were primarily young Asians. Only the frequently cited Polo [19] study took into account five young Caucasian patients aged 16–23 years. Thus, a single prospective study with 15 patients was finally included [20]. It showed a beneficial effect of the toxin with an 85% decrease in exposed gingival height.
Salivary pathologies
There are four salivary pathologies for which botulinum toxin treatment is useful: overdrooling, fistulas, sialoceles, and Frey syndrome.
Overdrooling
Ninety articles and studies dealt with this subject, but only 13 studies were selected for their high scientific merit according to HAS. These articles and studies were mainly dealing with hypersalivation in five patients with cerebral palsy [21–25] (Tab. III) and eight patients with Parkinson's disease [26–33] (Tab. IV).
The results of the literature review were unanimous: botulinum toxin has a significant effect on decreasing salivation and improving patients' quality of life. All studies analyzed confirmed the efficacy of the toxin on overdrooling, with a high level of evidence.
Critical analysis of the literature on the efficacy of botulinum toxin in the management of overdrooling in children with cerebral motor problems.
Critical analysis of the literature on the efficacy of botulinum toxin in the management of hypersalivation in adults with neuro-degenerative neurological diseases.
Fistulas and Sialoceles
A dozen of detailed clinical case studies reported the positive results in the use of botulinum toxin in the management of sialoceles and salivary fistulas. However, the Arnaud et al's case series [34], which included five cases with post-traumatic parotid complications that were initially untreated and that could not be effectively treated with botulinum toxin, provided opposing evidence. Therefore, there is no consensus regarding the effectiveness of this toxin for the treatment of salivary fistulas and sialoceles, which seems to be difficult to treat.
Frey's syndrome
Although 45 clinical case reports illustrated the use of botulinum toxin in the management of Frey's syndrome, only a single well-designed study was conducted to confirm the effiency of the toxin for this conditions [35].
Discussion
Muscular pathologies
Bruxism
Bruxism is a repetitive jaw muscles activity characterized by dental tightening or grinding, it may be diurnal or nocturnal [48]. Severe cases are extremely difficult to treat, and can cause depression and somatization due to debilitating chronic pain. This parafunction has a harmfull impact on surrounding structures (Fig. 1). The main objective when using botulinum toxin is therefore to limit chronic pain and to prevent dental dysfunction symptoms.
The positive effect of this toxin is found in the majority of the selected studies, and four out of the eight studies [5,6,9,10] showed a significant decrease in chronic pain. This led to our conclusion that efficient in bruxism treatment. However, these studies are heterogeneous: the patient recruitment procedure, the funding of these studies, and ethical issues are not necessarily comparable among the studies. Qerama [6], Göbel [9], and Benecke [10] have included exclusion criteria concerning analgesic administration in addition to botulinum toxin injection treatment, unlike Lew [5] and Ernberg [7]. The other studies did not mention this aspect, perhaps because of the difficulty of avoiding the use of associated analgesic administration in the context of chronic pain. The coadministration of these analgesics can indeed influence the results [7].
Therefore, the literature analysis justifies the use of botulinum toxin treating myofascial pains, but reservations must be expressed because of the heterogeneity of the currently available studies.
Injections are usually made exclusively in the masseter muscle: there isn't indeed any consensus regarding a combination of injections in both master and temporalis muscles (Fig. 2). A masseter palpation leads easily to muscle identification. When injecting the masseteric neurovascular bundle and the facial nerve should be avoided. The artery and the vein penetrate the facial bones anterior to the masseter. Muscles margins can be marked by using a surgical skin pen while asking the patient to contract the jaw (Fig. 3). Regarding injections in the temporalis muscle, bone, are the main elements to avoid the superior temporal artery and its venous branch, passing anterior to the ear and medial to the temporal bone. The main risk is injection into the neurovascular bundle.
 |
Fig. 1 Clinical results of bruxism in three patients. |
 |
Fig. 2 Injection in masseter in a patient with bruxism (credit: Dr. Chikhani). |
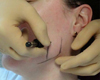 |
Fig. 3 Masseter muscle palpated and marked before injecting botulinum toxin into a patient with bruxism (credit: Dr. Chikhani). |
Masseter hypertrophy
Masseter hypertrophy is a symmetrical or asymmetrical masseter hypertonicity due to nocturnal bruxism or excessive chewing [36]. This muscular hyperactivity generates chronic muscular pain and a facial disharmony. On a long term, it causes deleterious effects on natural teeth and restored teeth (directly or indirectly).
Despite a lack of scientific evidence, many authors administer these injections to relieve patients when first-line treatments (anti-inflammatory drugs, muscle relaxants, opioids, or a surgical partial excision of the masseter muscle) [49] do not provide relief (Fig. 4).
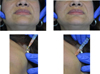 |
Fig. 4 Masseter injection in a patient with masseter hypertrophy (credit: Dr. Chikhani). |
Mouth Opening Limitation
MOL refers to a mouth contracture a result of painful position in the surrounding structures. Mouth opening in healthy adults is 47 ± 7 mm. MOL is considered mild when mouth opening is 20 mm, moderate when it is 20–40 mm, and severe when it is <10 mm [37]. Apart from pain, it leads to difficulties in chewing and phonation. The treatment is similar to the one in the initial pathology, but the muscle relaxing action of the toxin has an analgesic effect and functional benefits.
The purpose of the selected studies was to study the effect of botulinum toxin in treating muscular etiologies for MOL. The effect of the toxin was compared to injection in saline solution. Guarda-Nardini [15] was the only one to compare injection of the toxin with muscular manipulations. The originality of the latter study was the comparison between a single injection of Dysport® and muscular manipulation techniques (facial, neck, and
shoulder bones) developed by Dr. Stecco. However, these manipulations led to a bias due to dependent practitioners, according to the experiments carried out by Dr. Stecco. The analgesic effect can also be explained by the relaxing and reassuring patient–practitioner relationship that was created during these sessions. Denglehem's prospective study [17] focused on patient's living conditions. A less painful treatment option allows the realisation of impression in order to obtain orthodontic gutters. These impressions are sometimes impossible to obtain not only because of the oral limitations but also because of the pain felt by patients. Therefore, botulinum toxin is efficient in relieving secondary chronic muscular lockjaw. Nevertheless, the practitioner will have to manage the primary pathology for a complete cure.
Hypertonicity of the levator labii superioris muscles
The hypertonicity of the levator labii superioris muscles is better known as gingival smile, canine smile, or “gummy smile.” It is an excessive gingival smile that exposes >3 mm of gum [38]. The management of this condition is essentially orthodontic; however, aesthetic aspects are subjective and sometimes and sometimes a powerful musculature cannot allow a good smile line restoration. In most cases, the muscular hyperactivity of the upper lip is not detected. However, when existing and not being treated, a recurrence of the gingival smile is inevitable. The use of botulinum toxin has been proposed as a remedy, in addition to speech therapy exercises to ensure effective rehabilitation of the musculature.
Among the selected studies, only the frequently cited Polo study [19] included five young Caucasian patients aged 16–23 years. However, in this series of case studies, the selection criteria were imprecise and the author does not conduct any statistical analysis.
The Suber team [20] showed the toxin beneficial effect with with an 85% decrease in the exposed gingival height. However, the judgment criterion “>2 mm of exposed gum” did not correspond to the established definition of gingival smile, which is >3 mm of exposed gum. Similarly, the procedure, materials, and methods were based on a photographic comparison, which included a significant bias, and no statistical analysis was conducted. In conclusion, there is no clinical evidence regarding the use of botulinum toxin in the treatment of a gingival smile. Grade-A (Level-1) studies are therefore necessary to make objective conclusions on the efficacy of botulinum toxin in this indication.
Salivary pathologies
Acetylcholine interacts with presynaptic metabotropic cholinergic muscarinic receptors, which results in the release of postsynaptic salivary granules. Because of its effect on acetylcholine presynaptic receptors, botulinum toxin has a dose-dependent antisecretory action [39]. This is the same blocking mechanism described in neuromuscular junctions. The cholinergic nerve is therefore the target of botulinum toxin. By damaging the secretory circuit, it decreases or blocks the secretion of excessively active or aberrant glands [40].
overdrooling
Sialorrhea or hypersalivation is a social disability. Excess saliva affects the quality of life and patients oral health in a significant way [40]. Social rejection is considerable and can lead to social distress and depression in the most serious cases. Beyond the psychological aspect, ptyalicism promotes Candida infections through localized maceration. The authors also describe difficulties when eating, drinking, or talking [21]. The most commonly found etiology is cerebral palsy, or the sequelae of neurodegenerative diseases such as Parkinson's disease or amyotrophic lateral sclerosis. The control of etiological factors is not sufficient to effectively treat hypersialorrhea. Complementary therapies are also not completely satisfactory. The authors list many devices that can improve swallowing and tongue position [40–42]. Atypical swallowing is mainly related to neurological disorders caused by the previously mentioned pathologies. The results in the literature are unanimous; botulinum toxin has a significant effect on decreasing salivation and improving patient quality of life. All studies included confirmed with a high level of evidence that the toxin is effective in overdrooling cases.
Fistulas and Sialoceles
Salivary fistulas and sialoceles represent complications resulting from common parotid injuries. A salivary fistula is caused by the external drainage of saliva appearing initially or after a sialocele has developed enough to cause cutaneous pain [34]. The sialocele corresponds to an inflammatory pseudocapsule that contains salivary secretions within the cheek tissues that does not have proper drainage [34]. These complications compromise flap healing and increase sepsis risk.
On the other hand, interest in second-line surgeries seems limited [43]. Postoperative fibrosis increases the operational difficulty and therefore increases the risk of nerve or periglandular lesions. Moreover, treatments using atropine cannot target a specific gland. Atropine acts on all salivary glands causing xerostomia, which is unpleasant for patients. Use of botulinum toxin seems promising since it exposed the patient to few side effects. Should those appear, they transient and bearable.
Thus, many clinical cases reported promising results, but the evidence is limited so we cannot conclude on the efficacy of the toxin in the treatment of salivary fistulas and sialoceles.
Frey's syndrome
Frey's syndrome combines hyperhidrosis and cervicofacial erythema, which can occur during eating or following trauma to the ipsilateral parotid region [44]. It was described by Lucie Frey, a Polish neurologist in 1923 [45]. It is a postoperative complication of the parotid, but this syndrome can occur without any surgical trauma, as seen in cases of trigeminal herpes, parotitis, condylar fractures, and forceps trauma [46].
The main symptoms are sweating, redness, and significant heat of the precervical and cervical region, followed by taste sensations, real or imagined by the patient. This syndrome is thus called “gustatory sweating” in the English literature [44]. Patients describe the condition as a social disability and sometimes report pain.
The pathophysiology hypothesis is an aberrant cross-axonal regeneration. When trauma occurs, the auriculotemporal or intraparotid facial nerve may have been damaged. In attempting to restore nerve fiber regrowth, parotid secretory parasympathetic fibers may have regenerated along the cervicofacial sudoriparous sympathetic fibers [45]. Given these aberrant nerve connections, the sweat glands become involuntarily active. Erythema and sweating then occur when salivation is stimulated. It is therefore an iatrogenic neurological disorder [47].
The use of botulinum toxin topically, which blocks salivary stimulation appears to be a noninvasive therapeutic option. The use of this toxin that does not have a Pre market approval (PA) for this indication. It is based on the scientifically proven results obtained with the latter on axillary hyperhidrosis, a condition whose pathophysiology is similar [48].
The confirmation of this syndrome requires the completion of a Minor test dating from 1928. This is a colorimetric test that can highlight the affected areas. The cervicofacial skin is stained with an iodine solution (iodide: 1.5 g, castor oil 10 g, 95% alcohol 125 ml QSP) then sprinkled with flour. Salivation is stimulated by chewing a piece of apple or lemon. When chlorine secreted in sweat comes into contact with the iodide ions, it causes the brown color of the iodide to turn the skin violet. This is a quick and easy clinical test [44] (Figs. 5 and 6).
Although the study by Nolte [35] was randomized, it was not controlled. It compared two different doses of botulinum toxin, but no comparison was made with a control group. In addition, it did not use a blinded protocol, which means this test had a low level of evidence. Finally, the results were based on a nonreproducible subjective judgment criterion and the low number of individuals included was not representative. The efficiency of botulinum toxin for treating Frey's syndrome is therefore debatable. Therefore, it is not possible to reach a conclusion on the use of botulinum toxin in the treatment of this syndrome. However, all clinical cases and the Nolte study suggest that there are promising results in terms of its use. A study of high scientific merit with a sufficient number of subjects is indispensable for making a decision on this matter.
 |
Fig. 5 A 36-year-old patient after total parotidectomy for a pleomorphic adenoma. Minor test, A: painting with Betadine®, B: starch and salivary stimulation and C: evidence of Frey's syndrome (credit: Dr. Chikhani). |
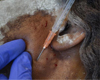 |
Fig. 6 A 36-year-old patient after total parotidectomy for a pleomorphic adenoma. Multiple injections (credit: Dr. Chikhani). |
Conclusion
The summary of scientific data concerning the orofacial indications on the use of botulinum toxin does not allow for a definitive conclusion. The muscle relaxant and antisecretory action of this toxin has been demonstrated in cases of bruxism, MOL, and hypersialorrhea. On the other hand, there are no medical benefits supported by evidence for masseter hypertrophy, hypertonicity of the levator labii superioris muscles, sialocele fistulas, or Frey's syndrome.
Additional high-level evidence studies, such as randomized controlled trials without major biases, are therefore necessary to eliminate the uncertainties that persist on the clinical impact of this toxin and to justify the establishment of evidence-based good practice recommendations. Like many medicines, the use of botulinum toxin is subject to a number of regulations and laws. Many therapeutic indications for these injections fall within the framework of a marketing authority.
In many cases, the reality shows the use of drugs outside Pre market approval (PA). In this context and under the freedom of prescription (the Code of Ethics Article R4127-238), the prescribing practitioner is liable and must not place their patient at an unnecessary risk.
To date, according to article R. 5124-2 of the Code of Public health, only pharmacists and hospital staff are allowed to dispense botulinum toxin after prescription by doctors qualified in plastic surgery and aesthetic reconstructive surgery, dermatology, cervicofacial surgery, maxillofacial surgery, and ophthalmology. These specialists may only prescribe and inject the toxin in mainly a hospital environment. Odontologists still are not authorized to use botulinum toxin.
Conflicts of interests
The authors declare that they have no conflicts of interest in relation to this article.
References
- Poulain B, Lonchamp E, Jover E, Popoff MR, Molgó J. Mechanisme d'action de la toxine botulique. Ann Dermatol Vénéréologie 2009;136:S73–S76. [CrossRef] [Google Scholar]
- Wenzel R. Pharmacology of botulinum neurotoxin serotype A. Am J Health Syst Pharm AJHP 2004;61:S5–S10. [Google Scholar]
- Dorizas A, Krueger N, Sadick NS. Aesthetic uses of the botulinum toxin. Dermatol Clin 2014;32:23–36. [CrossRef] [PubMed] [Google Scholar]
- Frevert J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs RD 2015;15:1–9. [CrossRef] [Google Scholar]
- Lew HL, Lee E, Castaneda A, Klima R, Date E. Therapeutic use of botulinum toxin type A in treating neck and upper-back pain of myofascial origin: a pilot study. Arch Phys Med Rehabil 2008;89:75–80. [CrossRef] [PubMed] [Google Scholar]
- Qerama E, Fuglsang-Frederiksen A, Kasch H, Bach FW, Jensen TS. A double-blind, controlled study of botulinum toxin A in chronic myofascial pain. Neurology 2006;67:241–245. [CrossRef] [PubMed] [Google Scholar]
- Ernberg M, Hedenberg-Magnusson B, List T, Svensson P. Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: a randomized, controlled, double-blind multicenter study. Pain 2011;152:1988–1996. [CrossRef] [PubMed] [Google Scholar]
- Ojala T, Arokoski J-P-A., Partanen J. The effect of small doses of botulinum toxin a on neck-shoulder myofascial pain syndrome: a double-blind, randomized, and controlled crossover trial. Clin J Pain 2006;22:90–96. [CrossRef] [PubMed] [Google Scholar]
- Göbel H, Heinze A, Reichel G, Hefter H, Benecke R. Dysport myofascial pain study group. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: results from a randomized double-blind placebo-controlled multicentre study. Pain 2006;125:82–88. [Google Scholar]
- Benecke R, Heinze A, Reichel G, Hefter H, Göbel H. Dysport myofascial pain study group. Botulinum type A toxin complex for the relief of upper back myofascial pain syndrome: how do fixed-location injections compare with trigger point-focused injections? Pain Med 2011;12:1607–1614. [Google Scholar]
- Guarda-Nardini L, Manfredini D, Salamone M, Salmaso L, Tonello S, Ferronato G. Efficacy of botulinum toxin in treating myofascial pain in bruxers: a controlled placebo pilot study. Cranio J Craniomandib Pract 2008;26:126–135. [Google Scholar]
- Lee S-J., McCall W-D., Kim Y-U., Chung S-C., Chung J-W. Effect of botulinum toxin injection on nocturnal bruxism: a randomized controlled trial. Am J Phys Med Rehabil Assoc Acad Physiatr 2010;89:16–23. [CrossRef] [PubMed] [Google Scholar]
- Fedorowicz Z, van Zuuren E, Schoones J. Botulinum toxin for masseter hypertrophy. Cochrane Database Syst Rev 2013;9:C D007510. [Google Scholar]
- Fietzek UM, Kossmehl P, Barthels A, Ebersbach G, Zynda B, Wissel J. Botulinum toxin B increases mouth opening in patients with spastic trismus. Eur J Neurol 2009;16:1299–1304. [CrossRef] [PubMed] [Google Scholar]
- Guarda-Nardini L, Stecco A, Stecco C, Masiero S, Manfredini D. Myofascial pain of the jaw muscles: comparison of short-term effectiveness of botulinum toxin injections and fascial manipulation technique. Cranio J Craniomandib Pract 2012;30:95–102. [Google Scholar]
- Kurtoglu C, Gur O-K., Kurkcu M, Sertdemir Y, Guler-Uysal F, Uysal H. Effect of botulinum toxin-A in myofascial pain patients with or without functional disc displacement. J Oral Maxillofac Surg 2008;66:1644–1651. [CrossRef] [PubMed] [Google Scholar]
- Denglehem C, Maes J-M., Raoul G, Ferri J. Toxine botulinique de type a: traitement antalgique des dysfonctions de l'appareil manducateur. Rev Stomatol Chir Maxillofac 2012;113:27–31. [CrossRef] [PubMed] [Google Scholar]
- Sidebottom A-J., Patel A-A., Amin J. Botulinum injection for the management of myofascial pain in the masticatory muscles. A prospective outcome study. Br J Oral Maxillofac Surg 2013;51:199–205. [CrossRef] [Google Scholar]
- Polo M. Botulinum toxin type A in the treatment of excessive gingival display. Am J Orthod Dentofacial Orthop 2005;127:214–218. [CrossRef] [PubMed] [Google Scholar]
- Suber J-S, Dinh T-P, Prince M-D, Smith P-D. OnabotulinumtoxinA for the treatment of a gummy smile. Aesthetic Surg J Am Soc Aesthetic Plast Surg 2014;34:432–437. [CrossRef] [Google Scholar]
- Reid SM, Johnstone BR, Westbury C, Rawicki B, Reddihough DS. Randomized trial of botulinum toxin injections into the salivary glands to reduce drooling in children with neurological disorders. Dev Med Child Neurol 2008;50:123–128. [CrossRef] [PubMed] [Google Scholar]
- Alrefai AH, Aburahma SK, Khader YS. Treatment of sialorrhea in children with cerebral palsy: a double-blind placebo controlled trial. Clin Neurol Neurosurg 2009;111:79–82. [CrossRef] [PubMed] [Google Scholar]
- Jongerius P-H, van den HF, van Limbeek J, Gabreëls F-J, van Hulst K, Rotteveel J-J. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics 2004;114:620–627. [CrossRef] [PubMed] [Google Scholar]
- Suskind D-L., Tilton A. Clinical study of botulinum-A toxin in the treatment of sialorrhea in children with cerebral palsy. The Laryngoscope 2002;112:73–81. [CrossRef] [PubMed] [Google Scholar]
- Basciani M, Di Rienzo F, Fontana A, Copetti M, Pellegrini F, Intiso D. Botulinum toxin type B for sialorrhoea in children with cerebral palsy: a randomized trial comparing three doses. Dev Med Child Neurol 2011;53:559–564. [CrossRef] [PubMed] [Google Scholar]
- Mancini F, Zangaglia R, Cristina S, Sommaruga M-G., Martignoni E, Nappi G, et al. Double-blind, placebo-controlled study to evaluate the efficacy and safety of botulinum toxin type A in the treatment of drooling in parkinsonism. Mov Disord 2003;18:685–688. [CrossRef] [PubMed] [Google Scholar]
- Guidubaldi A, Fasano A, Ialongo T, Piano C, Pompili M, Mascianà R, et al. Botulinum toxin A versus B in sialorrhea: a prospective, randomized, double-blind, crossover pilot study in patients with amyotrophic lateral sclerosis or Parkinson's disease. Off J Mov Disord Soc 2011;26:313–319. [CrossRef] [Google Scholar]
- Chinnapongse R, Gullo K, Nemeth P, Zhang Y, Griggs L. Safety and efficacy of botulinum toxin type B for treatment of sialorrhea in Parkinson's disease: a prospective double-blind trial. Mov Disord 2012;27:219–226. [CrossRef] [PubMed] [Google Scholar]
- Dogu O, Apaydin D, Sevim S, Talas D, Aral M. Ultrasound-guided versus blind intraparotid injections of botulinum toxin-A for the treatment of sialorrhoea in patients with Parkinson's disease. Clin Neurol Neurosurg 2004;106:93–96. [CrossRef] [PubMed] [Google Scholar]
- Lipp A, Trottenberg T, Schink T, Kupsch A, Arnold G. A randomized trial of botulinum toxin A for treatment of drooling. Neurology 2003;61:1279–1281. [CrossRef] [PubMed] [Google Scholar]
- Lagalla G, Millevolte M, Capecci M, Provinciali L, Ceravolo M-G. Botulinum toxin type A for drooling in Parkinson's disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2006;21:704–707. [Google Scholar]
- Kalf J-G., Smit A-M., Bloem B-R., Zwarts M-J., Mulleners W-M., Munneke M. Botulinum toxin A for drooling in Parkinson's disease: a pilot study to compare submandibular to parotid gland injections. Parkinsonism Relat Disord 2007;13:532–534. [CrossRef] [PubMed] [Google Scholar]
- Mazlan M, Rajasegaran S, Engkasan J-P, Nawawi O, Goh K-J, Freddy S-J. A double-blind randomized controlled trial investigating the most efficacious dose of Botulinum toxin-A for Sialorrhea treatment in Asian adults with neurological diseases. Toxins 2015;7:3758–3770. [CrossRef] [PubMed] [Google Scholar]
- Arnaud S, Batifol D, Goudot P, Yachouh J. Prise en chage non-surgical des plaies de la glande parotide et du canal de stenon: intérêt de la toxine botulique. Ann Chir Plast Esthét 2008;53:36–40. [CrossRef] [Google Scholar]
- Nolte D, Gollmitzer I, Loeffelbein DJ, Hölzle F, Wolff K-D. Botulinum toxin for treatment of gustatory sweating. A prospective randomized study. Mund- Kiefer- Gesichtschirurgie MKG 2004;8:369–375. [CrossRef] [Google Scholar]
- Majid OW. Clinical use of botulinum toxins in oral and maxillofacial surgery. Int J Oral Maxillofac Surg 2010;39:197–207. [CrossRef] [PubMed] [Google Scholar]
- Chassagne J-F, Cassier S, Simon E, Wang C, Chassagne S, Stricker C, et al. Limitation d'ouverture de bouche. EMC chirurgie orale et maxillo-faciale. Elsevier, 2009. [Google Scholar]
- Mazzuco R, Hexsel D. Gummy smile and botulinum toxin: a new approach based on the gingival exposure area. J Am Acad Dermatol 2010;63:1042–1051. [CrossRef] [PubMed] [Google Scholar]
- Devoize L, Dallel R. Salivation. In: EMC stomatologie. Paris: Elsevier Masson SAS, 2011. [Google Scholar]
- Monnier G, Tatu L, Parratte B, Cosson A, Michel F, Metton G. Hypersialorrhée, hypersudation et toxine botulique. Ann Réadapt Médecine Phys Rev Sci Société Fr Rééduc Fonct Réadapt Médecine Phys 2003;46:338–345. [Google Scholar]
- Friedman A, Potulska A. Quantitative assessment of parkinsonian sialorrhea and results of treatment with botulinum toxin. Parkinsonism Relat Disord 2001;7:329–332. [CrossRef] [PubMed] [Google Scholar]
- Meningaud J-P., Pitak-Arnnop P, Chikhani L, Bertrand J-C. Drooling of saliva: a review of the etiology and management options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:48–57. [CrossRef] [PubMed] [Google Scholar]
- Marchese-Ragona R, Marioni G, Restivo D-A., Staffieri A. The role of botulinum toxin in postparotidectomy fistula treatment. A technical note. Am J Otolaryngol 2005;27:221–224. [CrossRef] [Google Scholar]
- Laccourreye O, Muscatello L, Gutierrez-Fonseca R, Seckin S, Brasnu D, Bonan B. Syndrome de frey sévère post-parotidestomie: traitement par la neuro-toxine botulique de type A. Ann Oto-Laryngol Chir Cervico Faciale 1999;116:137–142. [Google Scholar]
- Moltrecht M, Michel O. The woman behind Frey's syndrome: the tragic life of Lucja Frey. The Laryngoscope 2004;114:2205–2209. [CrossRef] [PubMed] [Google Scholar]
- Cantarella G, Berlusconi A, Mele V, Cogiamanian F, Barbieri S. Treatment of Frey's syndrome with botulinum toxin type B. Otolaryngol Head Neck Surg 2010;143:214–218. [CrossRef] [PubMed] [Google Scholar]
- Ng S, Torjek C, Hovan A. Management of Frey syndrome using botulinum neurotoxin: a case report. ET J 2009;75:651–654. [Google Scholar]
- Lobbezoo F, Ahlberg J, Glaros AG, Kato T, Koyano K, Lavigne GJ et al. Bruxism defined and graded: an international consensus. J Oral Rehabil 2013;40:2–4. [CrossRef] [PubMed] [Google Scholar]
- Baş B, Ozan B, Muğlali M, Celebi N. Treatment of Masseteric hypertrophy with Botulinum toxin: a report of two cases. Med. Oral Patol. Oral Y Cirugía Bucal 2010;15:e649–e652. [Google Scholar]
All Tables
Critical analysis of the literature on the efficacy of botulinum toxin in the management of bruxism.
Critical analysis of the literature on the efficacy of botulinum toxin in the management of mouth opening limitation.
Critical analysis of the literature on the efficacy of botulinum toxin in the management of overdrooling in children with cerebral motor problems.
Critical analysis of the literature on the efficacy of botulinum toxin in the management of hypersalivation in adults with neuro-degenerative neurological diseases.
All Figures
 |
Fig. 1 Clinical results of bruxism in three patients. |
| In the text | |
 |
Fig. 2 Injection in masseter in a patient with bruxism (credit: Dr. Chikhani). |
| In the text | |
 |
Fig. 3 Masseter muscle palpated and marked before injecting botulinum toxin into a patient with bruxism (credit: Dr. Chikhani). |
| In the text | |
 |
Fig. 4 Masseter injection in a patient with masseter hypertrophy (credit: Dr. Chikhani). |
| In the text | |
 |
Fig. 5 A 36-year-old patient after total parotidectomy for a pleomorphic adenoma. Minor test, A: painting with Betadine®, B: starch and salivary stimulation and C: evidence of Frey's syndrome (credit: Dr. Chikhani). |
| In the text | |
 |
Fig. 6 A 36-year-old patient after total parotidectomy for a pleomorphic adenoma. Multiple injections (credit: Dr. Chikhani). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


