| Issue |
J Oral Med Oral Surg
Volume 26, Number 1, 2020
|
|
|---|---|---|
| Article Number | 5 | |
| Number of page(s) | 10 | |
| Section | Cas clinique et revue de la littérature / Up-to date review and case report | |
| DOI | https://doi.org/10.1051/mbcb/2019030 | |
| Published online | 07 January 2020 | |
Up-to Date Review And Case Report
Surgical-orthodontic treatment in patients with Ehlers–Danlos syndrome: a report of two familial cases
1
Unité de Pathologie-Chirurgie buccale, Pôle de Médecine et de Chirurgie bucco-dentaires, Hôpital Civil, Hôpitaux Universitaires de Strasbourg, Strasbourg, France
2
Pratique privée, Colmar, France
3
Université de Strasbourg, Faculté de Chirurgie Dentaire, Strasbourg, France
4
Unité d'Odontologie Pédiatrique, Pôle de Médecine et de Chirurgie bucco-dentaires, Hôpital Civil, Hôpitaux Universitaires de Strasbourg, Strasbourg, France
5
Centre de Référence des Manifestations Odontologiques des Maladies Rares, Pôle de Médecine et de Chirurgie bucco-dentaires, Hôpital Civil, Hôpitaux Universitaires de Strasbourg, Strasbourg, France
6
INSERM, UMR 1260, Regenerative Nanomedicine (RNM), FMTS, 11 rue Humann, Strasbourg 67000, France
* Correspondence: hchapuis.hc@gmail.com
Received:
27
August
2019
Accepted:
17
October
2019
Introduction: Ehlers–Danlos syndromes (EDS) are a group of rare inherited connective tissue disorders that affect the synthesis and structure of collagen in a ubiquitous manner. The clinical presentation can vary according to the associated genetic mutation. The 2017 international classification of EDS describes 13 types of EDS. Observation: The first part of this paper describes the surgical-orthodontic treatment for two sisters affected by a common and familial form of EDS, with a follow-up period of 8 years. The main symptoms were agenesis, impacted teeth, and delayed eruptions. Discussion: The second part proposes a review of oro-dental manifestations and discusses therapeutic approaches for patients with EDS. Conclusion: EDS can affect the oro-dental region with numerous consequences. Recognition of clinical symptoms and radiological signs is essential to provide appropriate dental care. Moreover, complete clinical and radiological assessment can allow early diagnosis of EDS.
Key words: Ehlers–Danlos / oral / dental / management
© The authors, 2020
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Ehlers–Danlos syndromes (EDS) include many genetic diseases with variable types of transmission, of penetrances and expressions that are characterized by an alteration of collagen, resulting in tissue damage and multiple visceral dysfunctions. Epidemiologically, the estimated incidence of EDS is between 1 per 5000 and 1 per 1,000,000 of births; this frequency varies according to the type of EDS and probably remains underdiagnosed [1,2].
The first clinical case of an EDS in the literature is attributed to the Danish dermatologist Edvard Ehlers in 1901. Seven years later, Henri-Alexandre Danlos, a French dermatologist, described the elasticity and skin fragility frequently associated with this pathology. In 1936, the name “Ehlers–Danlos syndrome” was proposed by the British dermatologist Frederick Parkes Weber [3].
The Villefranche clinical classification defined in 1997 six types of EDS characterized by predominant visceral involvement [4]. A more recent classification proposed by “the Ehlers–Danlos Society” in New York in 2017 and produced by an international consortium of experts defined 13 types of EDS corresponding to genetic mutations and therefore to specific clinico-molecular forms [2]. This new nomenclature widens the spectrum of genetic alterations associated with EDS and allows a better understanding of the molecular physiopathology of the different forms of EDS.
The diagnostic orientation is based on the presence of major and minor clinical criteria. [2,4]. Identifying the associated genetic mutation, the clinical phenotype and its mode of intra-familial transmission can help to improve the diagnostic accuracy (Tabs. I and II). Knowledge of the physiopathological features of EDS is necessary for the oral management of patients. Furthermore, the oral manifestations of EDS may constitute a clinical sign and allow for the early diagnosis and management of this condition and therefore can improve the prognosis in these patients. The oral management of two sisters with EDS within a familial context illustrates the clinical features and difficulties encountered.
Observations
Two sisters (patients A and B) who were initially suspected with EDS were followed up for 8 years in the Oral Surgery unit at the Strasbourg University Hospitals. Their initial checkup will be referred to from this point on as T0 (Fig. 1).
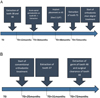 |
Fig. 1 Timeline and follow-up of patients A and B. |
Familial medico-surgical history
The main familial history of these patients consisted in multiple hemorrhagic events in the maternal line: repeated bleeding in the mother, frequent gingivorrhagia in the maternal grandmother, death by hemopneumomediastinum following gastroscopy in the maternal great-grandmother. A maternal uncle had a marfanoid appearance associated with generalized joint hyperlaxity. The paternal line was free of symptoms. All the above elements indicated a disease with autosomal dominant intra-familial transmission on the maternal lineage.
The mother of the two patients presented with an agenesis of tooth 35 and reported multiple root dilacerations observed after dental extractions. Recurring and former joint dysfunctions were noted in the latter.
Both patients and their mother were diagnosed with EDS within 1 year after the start of their follow-up by a national reference center for EDS. It was based on family history and the clinical signs described below. However, the genetic mutations of the two patients were not identified, making it impossible to identify the molecular subtype of EDS.
Patients' medical history
Patient A was 16 years old at the beginning of the follow-up (defined as T0). The main medical history was repeated predominantly distal joint dysfunctions; multiple bleeding manifestations: extradural hematoma at 1 year of age following minor trauma, spontaneous bruising ovarian hemorrhagic rupture; and various dermatological conditions (recurrent labial herpes, psoriasis, and alopecia).
Patient B was aged 10 years at T0 and also reported multiple and repeated joint dysfunctions (spontaneous sprains and dislocations of the shoulders, wrists and knees), recurring bleeding events (metrorrhagia, gingivorrhagia, and spontaneous bruising), diffuse chronic pain, proprioception disorders, epiphyseal detachment, myopia with astigmatism, acute intestinal invagination at 15 months of age, repeated mesenteric adenolymphitis, and penicillin allergy.
Oral management of patient A
At the beginning of the follow-up, the clinical examination and the initial radiological assessment showed agenesis of the two first maxillary premolars and of the right second mandibular premolar, a delay in the eruption of the left second mandibular premolar, and an odontogenic dentigerous cyst associated to the germ of the left lower wisdom tooth (38). Additionally, temporary teeth 75 and 85 persisted on the arch and the presence of the four germs of the wisdom teeth was noticed (Fig. 2A).
Management began with the extraction of tooth 38 and the surgical removal of the associated dentigerous cyst. The germ of tooth 48 was extracted subsequently.
For tooth 35, an initial therapeutic abstention with clinical and radiological monitoring was indicated because of a physiological root resorption of tooth 75, the asymptomatic nature of this inclusion, the absence of any anatomical obstacles, and the proximity of the eruption path. These characteristics indicated a positive evolution of the germ.
Three and a half years later, tooth 85 developed a periapical lesion of endodontic origin despite satisfactory conservative treatment, thereby indicating its extraction (Fig. 2B). For the agenesis of tooth 45, a cone beam computed tomography (CBCT) performed 6 months after the extraction of tooth 85 showed a good post-extraction bone healing. Implant prosthetic rehabilitation was offered to the patient with a favorable risk-benefit ratio despite the absence of extensive data about osseointegration in the context of EDS. Accordingly, a 3.8 × 8 mm XiveS* implant (Dentsply-Sirona, Manheim, Germany) was placed at T0 + 4 years with no postoperative complications. Radiological and clinical examinations confirmed the appropriate osseointegration of this implant, thereby allowing the placement of an abutment and a ceramic-metallic crown 4 months thereafter (Fig. 2C). Radiological monitoring showed bone-level stability at 29 months post-implantation (Fig. 2E). At T0 + 6 years, acute pulpitis encouraged the decision to perform emergency extraction of tooth 75 (Fig. 2D). Following the extraction of tooth 75 and after a thinking period the patient wanted to resume orthodontic treatment with the use of removable appliance in order to obtain orthodontic movement of tooth 35 onto the arch (Fig. 2E).
At T0 + 8 years, the last clinical control was satisfactory, with a slow evolution tooth 35. The implant of tooth 45 was asymptomatic at 3 years after implantation at the crestal bone level located 1 mm below the implant–abutment junction (Fig. 2F).
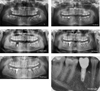 |
Fig. 2 Patient A; 8 years after radiological follow-up. (A) Initial panoramic X-ray. Agenesis of the upper first premolars and second lower right premolar, delayed eruption of tooth 35, remaining teeth 75 and 85, unerupted 4 wisdom teeth, and follicular cyst involving tooth 38. (B) Panoramic X-ray after 48 months of follow-up. Tooth 85 presented a periapical lesion. This chronic infection required tooth extraction. (C) Panoramic X-ray after 57 months of follow-up. Two months post-implantation (XiveS*, 3,8*8 mm, Dentsply-Sirona, Manheim, Germany) for replacement of tooth 45. (D) Panoramic X-ray after 70 months of follow-up (1.5 years after implantation). Peri-implant bone level stability and successful osteointegration. (E) Panoramic X-ray after 87 months of follow-up (29 months after implantation). Peri-implant bone level stability and successful osteointegration. Tooth 35 was erupting in good position. (F) Retroalveolar X-ray after 95 months of follow-up for implant 45 (3 years post-implantation). Peri-implant bone level stability under the level of the first thread. |
Oral management of patient B
Patient B, who was 6 years younger, was followed up 2 years after her sister (T0 + 25 months) and was 12 years old at this time. She presented neither agenesis, nor intraosseous maxillary or mandibular lesions. The germs of the four wisdom teeth were present, but tooth 37 was impacted with malposition and mesioversion (Fig. 3A).
A delay in the eruption of teeth 35 and 45 was noted, and tooth 37 did not make its eruption in orthoposition. Orthodontic treatment with fixed appliances (brackets) was performed for teeth 46 and 36 to make space for teeth 45 and 35. The decision to extract tooth 37 was made to promote the eruption of tooth 38 in its place (Fig. 3B).
At 4 years of follow-up, tooth 45 was placed on the arch but tooth 35 showed no development (Fig. 3C). CBCT demonstrated a horizontal and distal inclusion with a root curvature that would lead to complications for spontaneous eruption linked to close anatomical relationships between the germ and the mesial root of tooth 36. In agreement with the patient's orthodontist, a follow-up was suggested with the indication of an orthodontic-surgical procedure option if necessary. After an additional 18 months of observation, the development was favorable, with tooth 35 emerging on the arch and persistence of a marked root curvature (Fig. 3D).
Regarding the mandibular wisdom teeth, the germ of tooth 48, which was initially radiologically monitored in the absence of any complications, was extracted after 4 years of follow-up owing to right posterior mandibular crowding (Fig. 3D). The surgical clearance of tooth 38 for orthodontic traction was performed at the same time and was covered by clindamycin antibioprophylaxis with a posology of 1200 mg daily for 7 days starting the day before the procedure. Local analgesia of the germ of tooth 48 was performed using a solution of articaine 4% + adrenaline 1/2,00,000 by slow injection; however, the appropriate anesthetic clinical conditions for the procedure were not fully obtained. Anesthesia had to be readministered several times, the patient having already experienced similar anesthetic difficulties during a previous oral intervention. The operative course was unusual and marked by the occurrence of severe toxidermy related to infectious mononucleosis and most likely induced by antibiotic treatment. This viral infection required a short stay in a dermatology department. The development was favorable after a symptomatic treatment with topical corticosteroids. Significant postoperative pain was experienced at the operative site of tooth 48, which was associated with a dry socket.
At 6 years after the beginning of the treatment, the orthodontic treatment is in consolidation phase, and all the teeth are placed suitably on the arch (control radiography was not performed).
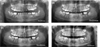 |
Fig. 3 Patient B; 4 years after radiological follow-up. (A) First panoramic X-ray (T0 + 25 months). The start of orthodontic treatment with delayed eruption of teeth 35, 45, and 37 tilted on the mesial side. Four wisdom teeth were unerupted. (B) Panoramic X-ray after 6 months of follow-up (T0 + 31 months). Tooth 37 was extracted. (C) Panoramic X-ray after 2 years of follow-up (T0 + 49 months). Tooth 45 was in a normal position, and tooth 35 was still unerupted. (D) Panoramic X-ray after approximately 3.5 years of follow-up (T0 + 69 months). Tooth 35 was in a normal position, and tooth 38 was surgically exposed for orthodontic procedures. Teeth 45 and 35 show a radicular hook apical region. |
Discussion
Diagnosis of EDS
Advances in research have identified many genetic mutations associated with EDS, which culminated in a new clinico-molecular classification in 2017 correlating genotype and phenotype. Nevertheless, the Villefranche clinical classification remains relevant and is still commonly used by clinicians [4]. Considering the global personal and family history of our two patients, they were affected by a common form of predominantly vascular EDS. EDS are mainly characterized by an alteration of collagen and therefore affect the body ubiquitously. It is a group of complex diseases that is further complicated by the multiplicity of existing forms and a broad spectrum of manifestations that can be associated with them (Tabs. I and II). Regarding patient treatment, a diagnosis of EDS was confirmed by a national reference center, but the exact subtype was not known because of the lack of an available molecular diagnosis. Indeed, the genetic heterogeneity of EDS can make it difficult to establish a molecular diagnosis by conventional direct sequencing of genes encoding the various isoforms of collagen. Therefore, next-generation sequencing could be recommended for these patients allowing a parallel analysis of many genes. One of the tools at our disposal is the Genodent DNA chip, which integrates several hundred genes involved in the molecular etiology of dental anomalies, including structural abnormalities and connective tissue abnormalities, and in particular COL1A1 and COL17A1 [5]. In the event of a negative DNA chip result, an exome analysis could be performed, consisting of a genome-wide exploration of all the coding sequences of the genome. This type of molecular analysis is more complex but can significantly increase the probability of identifying a pathogenic polymorphism. In the case of a negative exome analysis result, a study of non-coding and regulatory gene sequences can be conducted [5].
Patient care must be comprehensive and multidisciplinary to be effective by integrating all systemic and psycho-social aspects associated with EDS, including oral manifestations [6].
Oral characteristics described in the framework of Ehlers–Danlos syndromes
Periodontium
Patients with EDS are considered to present a high risk of periodontal disease [7]. This susceptibility of the periodontium is directly linked to the collagen abnormalities present in the supporting tissues of the tooth. The diffusion of nutrients and the activity of the immune cells are deficient, which explains the increased sensitivity to bacteria, altered tissue metabolism and a reduced potential for tissue healing. This higher risk of periodontal disease would be, however, only associated with periodontal EDS [8].
In a recent case-control study, Honoré et al. studied the most common oral characteristics found in a cohort of 26 patients with hypermobile-type EDS. Regarding the periodontal parameters, the authors found the plaque index to be significantly higher compared with the control group (mean, 0.396 vs. 0.059, p < 0.001). They also determined a greater attachment loss in the upper left and lower right upper molars and concluded that hypermobile-type EDS is associated with a potential predisposition to periodontal disease [9].
Soft tissues
The absence of the upper labial and/or lingual frenulum is frequently found in vascular, hypermobile, and classical EDS [10]. The Gorlin sign (ability to touch one's nose with the tip of the tongue) is also more common than in the general population (50% vs. 15%) [11]. However, these clinical signs, although evocative, are not pathognomonic of this syndrome, and prospective studies would be necessary to determine their possible intrinsic diagnostic value [9,12]. The presence of palatal petechial lesions is frequently observed in the context of EDS and other collagen pathologies [13].
Dental phenotype
The presence of dental agenesis and microdontia is frequent [14,15]. From a morphological point of view, the teeth frequently present root anomalies (dilaceration and fusion) and dysmorphic crown features (presence of prominent cusps in the premolars and molars) that can make orthodontic treatment more complex [14,15]. Some authors also report a lower coronary height in the central maxillary and mandibular incisors in patients with hypermobile EDS. This observation could be a consequence of the structural abnormalities of the connective tissue characteristic of this pathology or reflect greater attrition or associated dentine dysplasia [9]. Structural anomalies are also common. There is an increased frequency of coronary pulp calcifications, vascular pulp lesion, and aberrant dentine tubules. These root canal abnormalities can constitute obstacles and lead to per-operative complications during eventual endodontic treatment [15,16]. Patients affected by EDS present also a high individual caries risk and susceptibility to fractures due to specific structural enamel fragility associated with coronary morphological abnormalities [16,17].
Temporomandibular joints (TMJ)
Patients with EDS are frequently affected by generalized ligament hyperlaxity that does not spare the oro-facial sphere. Therefore, these patients have a significant risk of dislocations and subluxations of the temporomandibular joints and often have symptoms corresponding to cranio-mandibular dysfunction (joint locking, clicking, cracking, and localized or referred pain) [15]. These dysfunctions are more common and more complex in patients with hypermobile-type EDS [6,18].
Salivary function
Previous studies reported an increased prevalence of xerostomia and/or dry mouth sensation in patients with hypermobile-type EDS joint hypermobility. This observation may reflect the frequent multifactorial dysfunction frequently associated with EDS and indirectly increase the risk of caries [9,19].
Other observations
Sakar et al. reported two familial cases of gigantiform cementum associated with type VIII EDS. Because of the rarity of this type of lesion and the low prevalence of EDS, a possible common genetic etiology was considered [20].
Oral management of patients with EDS
General principles
The risk-benefit ratio of any procedure in patients with EDS should be particularly considered because of the increased risk of general complications (hemorrhagic, cutaneous, and joint) and oro-dental (endodontic and periodontal) complications and should ideally be discussed within a multidisciplinary consultation meeting. Informing patients about this risk is also important [8,16,21].
Irrespective of the procedure considered (conservative care or orthodontics or oral surgery), the patient's treatment must be optimal with suitable support, the waiting times and the duration of the sessions must be reduced to avoid the occurrence of cutaneous (ecchymoses or ischemia) and joint complications (dislocations and sprains) [14,16].
The main challenge in oral cares of patients with EDS is the preservation of a functional dentition as long as possible and prevention of any infectious pathology, which can be addressed by regular specialized follow-ups and implementation of adapted. Early preventive and conservative care and motivation for oral hygiene to prevent complex or potentially iatrogenic fixed or adjunctive prosthetic rehabilitation are also essential and integrated in the treatment plan [13,16,22].
Orthodontic care
Pre-therapeutic clinical and radiological analysis aims to diagnose any sign of endodontic or periodontal pathology associated with increased risk during treatment (root dilacerations, gingival recessions, and carious lesions). The orthodontic devices used must be simplified as much as possible in order to reduce the periodontal risk. Any element with potential for soft tissue trauma, e.g., hook and bracket, must be protected by a composite cap [23]. The tensile forces used are of low intensity to reduce the risk of subluxation or desmodontal microhemorrhage inherent in the weak periodontal ligaments in these patients [14,23]. Motivation for oral hygiene and regular periodontal follow-up are essential at each stage of treatment and should be continued. Clinical and radiological monitoring should be regularly performed to promptly detect any iatrogenic complications such as external apical root resorption and/or aggressive periodontolysis. Patients with subtypes IV (vascular) and VIII (periodontal) seem particularly vulnerable [24,25,26]. Following treatment, an orthodontic retainer is placed and will be maintained for a prolonged period of time because of the high risk of relapse [23,27].
Surgical management
Regarding the risk of infectious endocarditis associated with oral surgical procedures, patients with EDS are not considered to be at high risk and therefore would not benefit from preoperative antibiotic prophylaxis in a systematic manner [28].
Patients with EDS are considered at high risk of bleeding. The preparation of a preoperative hemostasis assessment is recommended. In vascular-type EDS, preoperative prescription of tranexamic acid or desmopressin should be considered to reduce the risk of peri- or postoperative bleeding events. Depending on the procedure considered, a pre-transfusion assessment may also be prescribed. Postoperative monitoring for 24 h and the proximity of a suitable emergency reception facility is recommended [21].
The fragility of the soft tissues and the risk of delayed mucosal healing require the most atraumatic surgical procedure, and closure without tension by a nonabsorbable suture thread can be left in place for a long time.
To reduce the risk of TMJ pain and/or dislocation, the range of mouth opening, forces exerted during tooth extractions, and the duration of the sessions should be limited [11,14].
Regarding dental extractions, postoperative complications are more frequent in patients with hypermobile-type EDS than in unaffected individuals. Reported complications include delayed mucosal healing, postoperative hemorrhagic events, and prolonged high-intensity pain [9].
Scientific data on implant procedures and osseointegration in patients with EDS are limited. Jensen et al. (2012) reported a series of 12 cases that were followed between 2 and 12 years after implant placement and found no implant loss, low crestal bone loss and a high level of patient satisfaction [29]. Rinner et al. (2018) reported a series of three familial cases of periodontal-type EDS with implant failures secondary to severe peri-implantitis despite appropriate periodontal management. The bone loss was amplified by the procedure, leading to the conclusion that there is a strong mechanical influence on the implant prognosis in these three patients [22]. Therefore, the biological mechanisms of osseointegration seem to be preserved without major and specific complications in the context of the EDS; however, clinical studies involving larger cohorts of patients are needed. The main problem consists in the maintenance and long-term prognosis of implants in these patients at high risk of periodontal disease. Furthermore, peri-implant soft tissue management must be given special attention from the first surgery because of the difficulty of secondary management by periodontal plastic surgery (connective tissue graft or local flap).
For anesthesia, there is no pharmacodynamic or pharmacokinetic contraindication in patients with EDS [21]. However, a study reported reduced efficacy of topical and para-apical anesthesia exclusively in hypermobile-type EDS, but did not determine any specific pathophysiological mechanism that may explain this observation [30]. To reduce this risk of failure, it is recommended to use an anesthetic solution containing adrenaline, which should be slowly injected at room temperature [15]. A neuro-sedative premedication and staggered care over several sessions would also be effective [9].
The anesthesia by an inferior alveolar nerve block is not recommended in vascular-type EDS because of the high risk of hemorrhagic stroke by vessel rupture [21]. In other types of EDS, the risk-benefit ratio of a loco-regional block should be evaluated for every patient. The use of ultrasound guidance may reduce the risk of hemorrhagic complications [21].
Management under general anesthesia can lead to other potentially serious iatrogenic complications (spontaneous peri-intubation visceral hemorrhagic rupture, postural cutaneous or articular injuries, and cardiac rhythm disorders, such as postural orthostatic tachycardia syndrome) [21]. The indication of general anesthesia should therefore be considered in the context of a prior multidisciplinary consultation (surgeon, anesthesiologist, and cardiologist) and does not reduce the risk of occurrence of peri- and postoperative complications.
Irrespective of the type of anesthesia or surgery considered, the prevention of nausea and vomiting by prescribing anti-emetics postoperatively is indicated to prevent the high risk of esophageal rupture which is potentially lethal in patients with EDS [21].
Pain treatment
Patients with EDS often suffer from various types of severe pain (articular, muscular, digestive, etc.), it is therefore necessary to adopt a reasonable attitude regarding the peri-procedural analgesic prescriptions. In a recent publication, the French Ehlers-Danlos Syndrome Study and Research Group (GERSED) summarized the various methods of analgesic management in this particular pathological context.
Common level 1 analgesics may be prescribed without restriction in case of low-intensity pain.
Regarding level 2 analgesics, the GERSED recommends the use of tramadol in sustained release form and for short periods in case of moderate pain. For the management of severe pain, it is recommended to use nefopam. Level 3 analgesics should be avoided because of the high risks of addiction. A complementary nonsteroidal anti-inflammatory prescription may be prescribed concurrently.
Muscular pain can be effectively treated with baclofen or L-carnitine. Lidocaine may also be useful in topical form in case of gum pain or in subcutaneous infiltrations near areas of musculo-articular dysfunction (cervico-occipital and dorsal regions frequently affected by dysfunctions of the manducatory apparatus).
Finally, maxillofacial physiotherapy with a re-educative and decontracturing aim, cognitive and behavioral therapies, or sophrology can also complement the previously mentioned pharmacological measures [6].
Conclusion
The dental management of patients with EDS must consider specific risks, both oral and general (articular, cutaneous, and visceral). Multidisciplinary consultation during implementation of the treatment plan and an evaluation of the risk-benefit ratios for the various procedures should be indicated in the context of the EDS. The majority of these patients can benefit from conservative, orthodontic, and surgical procedures, subject to certain precautions. The major challenge for practitioners is preservation of the oral status of patients who are considered at high risk of periodontal and endodontic diseases for as long as possible. Therapeutic education, motivation to maintain a high level of oral hygiene, and careful and regular specialized follow-up are therefore imperative. These measures would enable the prevention and prompt detection of the occurrence of oral complications to reduce the functional, esthetic, and psychological impact in these patients, who are frequently poly-pathological. Specialists in oral heathcare can also help with the early detection of EDS, a pathological entity that is frequently a source of diagnostic errors, by detecting certain anatomical and structural dental and mucosal features associated with them.
Conflicts of interests
The authors declare that they have no conflicts of interest in relation to the publication of this article.
Acknowledgments
The authors thank the patients and their families for their kind collaboration and participation.
References
- Orphanet, le portail des maladies rares et des médicaments orphelins. https://www.orpha.net/consor/cgi-bin/Disease_Search_Simple.php?lng=FR. Consulté le 4 octobre 2019 [Google Scholar]
- Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 International classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 2017;175: 8–26. [CrossRef] [PubMed] [Google Scholar]
- Parkes Weber F. Ehlers-Danlos Syndrome. JRSM 1936;30:30–31. [CrossRef] [Google Scholar]
- Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet 1998;77:31–37. [Google Scholar]
- Prasad MK, Geoffroy V, Vicaire S, Jost B, Dumas M, Le Gras S, et al. A targeted next-generation sequencing assay for the molecular diagnosis of genetic disorders with orodental involvement. J Med Genet 2016;53:98–110. [CrossRef] [PubMed] [Google Scholar]
- Groupe d'étude et de recherche du Syndrome d'Ehlers-Danlos (GERSED). Du diagnostic à la prize en charge du Syndrome d'Ehlers-Danlos hypermobile (SEDh). 2019. 88 pages. [Google Scholar]
- Hagberg C, Berglund B, Korpe L, Andersson-Norinder J. Ehlers-Danlos Syndrome (EDS) focusing on oral symptoms: a questionnaire study. Orthod Craniofac Res 2004;7:178–185. [PubMed] [Google Scholar]
- Kapferer-Seebacher I, Lundberg P, Malfait F, Zschocke J. Periodontal manifestations of Ehlers-Danlos syndromes: A systematic review. J Clin Periodontol 2017;44:1088–1100. [CrossRef] [PubMed] [Google Scholar]
- Honoré MB, Lauridsen EF, Sonnesen L. Oro‐dental characteristics in patients with hypermobile Ehlers‐Danlos Syndrome compared to a healthy control group. J Oral Rehabil 2019. [Google Scholar]
- Parrini S, Bellosi A, Barducci A, Biancardi G, Latini G, De Felice C. Abnormal Oral Mucosal Light Reflectance: A New Clinical Sign of Ehlers-Danlos Syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;97:335–338. [CrossRef] [PubMed] [Google Scholar]
- Abel MD, Carrasco LR. Ehlers-Danlos Syndrome: Classifications, Oral Manifestations, and Dental Considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:582–590. [CrossRef] [PubMed] [Google Scholar]
- Perrinaud A, Matos M, Maruani A, Mondon K, Machet L. Absence of inferior labial or lingual frenula in Ehlers-Danlos syndrome: a ornew diagnostic criterion? Ann Dermatol Venereol 2007;134:859–862. [CrossRef] [PubMed] [Google Scholar]
- Delarue M, Souche B, Cousty S, Vaysse F, Duran D. Syndrome d'Ehlers-Danlos: à propos d'un cas. J Oral Med Oral Surg 2010;16: 119–124. [CrossRef] [Google Scholar]
- Norton LA, Assael LA. Orthodontic and Temporomandibular Joint Considerations in Treatment of Patients with Ehlers-Danlos Syndrome. Am J Orthod Dentofacial Orthop 1997;111:75–84. [CrossRef] [PubMed] [Google Scholar]
- Mitakides J, Tinkle BT. Oral and Mandibular Manifestations in the Ehlers-Danlos Syndromes. Am J Med Genet C Semin Med Genet 2017;175:220–225. [PubMed] [Google Scholar]
- Delarue M. Syndrome d'Ehlers-Danlos et odontologie. J Rea Med 2016;36:85–88. [Google Scholar]
- Klingberg G, Hagberg C, Norén JG, Nietzsche S. Aspects on dental hard tissues in primary teeth from patients with Ehlers-Danlos syndrome. Int J Paediatr Dent 2009;19:282–290. [CrossRef] [PubMed] [Google Scholar]
- Diep D, Fau V, Wdowik S, Bienvenu B, Bénateau H, Veyssière A. Dysfonction de l'appareil manducateur et syndrome d'Ehlers-Danlos de type hypermobile : étude cas-témoin. Rev Stomatol Chir Maxillofac Chir Orale 2016;117:228–233. [PubMed] [Google Scholar]
- Bravo JF, Wolff C. Clinical study of hereditary disorders of connective tissues in a Chilean population: joint hypermobility syndrome and vascular Ehlers-Danlos syndrome. Arthritis Rheum 2006;54:515–523. [CrossRef] [PubMed] [Google Scholar]
- Sakar O, Aren G, Mumcu Z, Ünalan F, Aksalli N, Tolgay CG. Familial gigantiform cementoma with Ehlers-Danlos syndrome: a report of 2 cases. J Adv Prosthodont 2015;7:178–182. [CrossRef] [PubMed] [Google Scholar]
- Wiesmann T, Castori M, Malfait F, Wulf H. Recommendations for anesthesia and perioperative management in patients with Ehlers-Danlos syndrome(s). Orphanet J Rare Dis 2014;23: 109. [Google Scholar]
- Rinner A, Zschocke J, Schossig A, Gröbner R, Strobl H, Kapferer-Seebacher I. High risk of peri-implant disease in periodontal Ehlers–Danlos syndrome. A case series. Clin Oral Implants Res 2018;29:1101–1106. [PubMed] [Google Scholar]
- Cohen N, Cohen-Levy J. Painful hyperlaxity (Ehlers-Danlos Syndrome). J Dentofacial Anom Orthod 2014;18:112. [CrossRef] [EDP Sciences] [Google Scholar]
- Karrer S, Landthaler M, Schmalz G Ehlers-Danlos syndrome type VIII with severe periodontitis and apical root resorption after orthodontic treatment. Acta Derm Venereol 2000;80:56–57. [CrossRef] [PubMed] [Google Scholar]
- Badauy CM, Gomes SS, Sant'Ana Filho M, Chies JA. Ehlers-Danlos syndrome (EDS) type IV: review of the literature. Clin Oral Investig 2007;11:183–187. [CrossRef] [PubMed] [Google Scholar]
- Arun T, Nalbantgil D, Sayinsu K. Orthodontic treatment protocol of Ehlers-Danlos syndrome type VI. Angle Orthod 2006;76: 177–183. [Google Scholar]
- Fridrich KL, Fridrich HH, Kempf KK, Moline DO. Dental implications in Ehlers-Danlos Syndrome. A case report. Oral Surg Oral Med Oral Pathol 1990;69:431–435. [Google Scholar]
- AFFSAPS. Prescription des antibiotiques recommandations en pratique bucco-dentaire. 2011. https://ansm.sante.fr [Google Scholar]
- Jensen JL, Storhaug K. Dental implants in patients with Ehlers-Danlos syndrome: a case series study. Int J Prosthodont 2012;25:60–62. [PubMed] [Google Scholar]
- Hakim AJ, Grahame R, Norris P, Hopper C. Local anaesthetic failure in joint hypermobility syndrome. J R Soc Med 2005;98: 84–85. [CrossRef] [PubMed] [Google Scholar]
All Tables
All Figures
 |
Fig. 1 Timeline and follow-up of patients A and B. |
| In the text | |
 |
Fig. 2 Patient A; 8 years after radiological follow-up. (A) Initial panoramic X-ray. Agenesis of the upper first premolars and second lower right premolar, delayed eruption of tooth 35, remaining teeth 75 and 85, unerupted 4 wisdom teeth, and follicular cyst involving tooth 38. (B) Panoramic X-ray after 48 months of follow-up. Tooth 85 presented a periapical lesion. This chronic infection required tooth extraction. (C) Panoramic X-ray after 57 months of follow-up. Two months post-implantation (XiveS*, 3,8*8 mm, Dentsply-Sirona, Manheim, Germany) for replacement of tooth 45. (D) Panoramic X-ray after 70 months of follow-up (1.5 years after implantation). Peri-implant bone level stability and successful osteointegration. (E) Panoramic X-ray after 87 months of follow-up (29 months after implantation). Peri-implant bone level stability and successful osteointegration. Tooth 35 was erupting in good position. (F) Retroalveolar X-ray after 95 months of follow-up for implant 45 (3 years post-implantation). Peri-implant bone level stability under the level of the first thread. |
| In the text | |
 |
Fig. 3 Patient B; 4 years after radiological follow-up. (A) First panoramic X-ray (T0 + 25 months). The start of orthodontic treatment with delayed eruption of teeth 35, 45, and 37 tilted on the mesial side. Four wisdom teeth were unerupted. (B) Panoramic X-ray after 6 months of follow-up (T0 + 31 months). Tooth 37 was extracted. (C) Panoramic X-ray after 2 years of follow-up (T0 + 49 months). Tooth 45 was in a normal position, and tooth 35 was still unerupted. (D) Panoramic X-ray after approximately 3.5 years of follow-up (T0 + 69 months). Tooth 35 was in a normal position, and tooth 38 was surgically exposed for orthodontic procedures. Teeth 45 and 35 show a radicular hook apical region. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


