| Issue |
J Oral Med Oral Surg
Volume 24, Number 2, June 2018
|
|
|---|---|---|
| Page(s) | 60 - 62 | |
| Section | Cas clinique / Short case report | |
| DOI | https://doi.org/10.1051/mbcb/2017032 | |
| Published online | 29 June 2018 | |
Short Case Report
Oral ulcers in patients treated with palbociclib: a case report
Odontology Department, Pellegrin Hospital,
Bordeaux, France
* Correspondence: cedric.alande@laposte.net
Received:
12
November
2017
Accepted:
14
December
2017
Introduction: Palbociclib is an approved drug in the treatment of women with advanced or metastatic estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative, breast cancer. The purpose of this article is to present the first case ever described of oral ulcers caused by palbociclib. Observation: A case of drug-induced oral ulcers is reported. The patient was treated with a combination of palbociclib and Fulvestrant for a breast cancer relapse with pulmonary involvement. These ulcers were localized on the ventral face of the tongue and on the gum. A therapeutic combination of infrared laser biostimulation and a topical application of Dermoval® and Dynexan® were carried out. Palbociclib was concurrently discontinued. The lesions healed in about 15 days. Comments: Palbociclib is an inhibitor of cyclin-dependent kinases 4 and 6 (CDK 4/6). Thereby, it blocks many signaling pathways responsible for cell proliferation. This property weakens the oral mucosa and could be the cause of the observed ulcers. These latter having been particularly debilitating for the patient. Conclusion: Palbociclib-related oral aphthous ulcers are similar to those reported with other targeted therapies. The combination of infrared laser biostimulation and local corticosteroid therapy did not prevent the discontinuation of the cancer treatment.
Key words: palbociclib / ulcer / breast cancer
© The authors, 2018
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Palbociclib is a drug indicated in the treatment of locally advanced or metastatic, hormone receptor-positive, and human epidermal growth factor-2 (HER-2) receptor-negative breast cancer. Oral ulcers caused by palbociclib appear to be reported in phase-2 and phase-3 studies, but have never been specifically described, unlike other older targeted therapies such as mTOR inhibitors. The purpose of this report is to present the descriptive findings of the first reported case of oral ulcers attributable to palbociclib.
Observation
In 2017, a 62-year-old female consulted the oral surgery department for management of oral ulcers. According to her medical history she had been diagnosed with breast cancer and had received treatment in 1997. In 2016, she went into remission. In March 2017, several pulmonary nodules related to the mammary tumor were found. These were associated with an increase in the level of carbohydrate antigen 15-3 (CA 15-3). Chemotherapy treatment combining palbociclib (Ibrance®) and fulvestrant (Faslodex®) was initiated. Palbociclib was administered at a dosage of 125 mg/day for 21 out of 28 days. Oral ulcers occurred on the 15th day of treatment. However, the patient continued taking the palbociclib, which had proven somewhat effective as a decrease in CA 15-3 was observed. The other biological tests were normal, there was no associated neutropenia. During her first visit to the department, the patient complained of severe pain (visual analog scale = 9) associated with speech difficulties. A clinical examination revealed multiple, “cookie cutter” ulcers, ≤ 1 cm, on the dorsal side of the tongue (Fig. 1). A much larger, irregularly-shaped ulcer was also found on the vestibular gingiva of the tooth 46 (Fig. 1). These ulcers were shallow and had a fibrinous base with an erythematous border. This clinical picture was suggestive of iatrogenic oral ulcers attributable to the use of palbociclib. The patient underwent an infrared laser biostimulation session (4 J/cm2) (Fig. 2a). In addition, the patient had to apply a compound preparation to the lesions consisting of clobetasol (Dermoval®) and lidocaine hydrochloride (Dynexan®) in equal amounts. A follow-up consultation took place after 3 weeks. The patient did not report any immediate benefit from laser biostimulation. Meanwhile, because of the debilitating discomfort caused by the ulcers, palbociclib treatment was discontinued. A clinical examination showed that the lesions were healing (Fig. 2b). Only one unobtrusive gingival ulcer, similar in appearance to the previous ones, was present near tooth 23. An attempt was made to reintroduce a lower-dose treatment in the summer of 2017, but the ulcers recurred in a just few days, thereby suggesting that palbociclib was responsible for the initial occurrence of the ulcers.
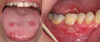 |
Fig. 1 Appearance of ulcers at the first consultation. |
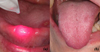 |
Fig. 2 (a) Laser biostimulation, (b) healing of the dorsal side of the tongue at 15 days. |
Discussion
Palbociclib is a drug indicated in the treatment of locally advanced or metastatic, hormone receptor-positive, and HER-2 receptor-negative breast cancer. It is used in combination with an aromatase inhibitor (ex: Letrozole®) or with Fulvestrant® in women who have been previously received hormone therapy. Its efficacy has been demonstrated in randomized phase-2 and phase-3 studies [1, 2]. The most common adverse side effects reported in patients receiving palbociclib in randomized clinical trials were neutropenia, infections, leukopenia, fatigue, nausea, stomatitis, anemia, alopecia, and diarrhea [2]. The term stomatitis refers here to aphthous ulcers, cheilitis, glossitis, oral ulcers, mucous membrane inflammations, and mouth pain. There is no case report of aphthous ulcers outside phase-2 and phase-3 studies. Finding ulcers on the dorsal side of the tongue is rare because mucositis caused by chemotherapy and targeted therapies is generally found on nonkeratinized mucous membranes. The physiopathological mechanisms responsible for the ulcers observed in the case presented are complex. Palbociclib is a selective and reversible inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6). However, CDK4/6 are downstream of multiple signaling pathways that control cell proliferation. The treatment of drug-induced oral ulcers, and mucositis more generally, is based primarily on prevention with regular follow-ups and rigorous oral hygiene. The treatment of mucositis under targeted therapies is comprised of local corticosteroid therapy combined with laser biostimulation. Although there is no consensus on the dose and frequency of sessions, low-level laser therapy treatments seem to play a part in the prevention and treatment of radiotherapy-induced and chemotherapy-induced mucositis [3]. They stimulate cell renewal by increasing ATP synthesis, accelerating mitosis, and stimulating local microcirculation. This treatment was attempted as a cure, but the patient did not report any improvement. However, palbociclib treatment was discontinued concomitantly with the laser biostimulation session; thus, it is difficult to determine exactly which factor was responsible for the disappearance of the ulcers.
Palbociclib was recently made commercially available (November 2016) and no similar cases are reported in the literature. Given the effectiveness of this drug demonstrated by many studies, its use is likely to increase in the coming years. In addition, new therapeutic indications are emerging: multiple myeloma treatment [4] and intracranial growing teratoma syndrome [5], which suggests a possible increase in the frequency of its adverse effects. In this scenario, oral surgeons must be made aware of its side effects.
Conclusion
Palbociclib, as a CDK4/6 inhibitor, appears to be an effective therapeutic tool in the treatment of locally advanced or metastatic, hormone receptor-positive and HER-2 receptor-negative breast cancers. Aphthous oral ulcers are a complication which led to the discontinuation of treatment despite the implementation of local corticosteroid therapy and laser biostimulation. The frequency and severity of mucositis secondary to palbociclib treatment remains to be clarified because the drug recently made available for purchase.
Conflict of Interest
The authors declare that they have no conflicts of interest in relation to this article.
References
- Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y, Thummala AR, Voytko NL, Fowst C, Huang X, Kim ST, Randolph S, Slamon DJ. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25–35 [CrossRef] [PubMed] [Google Scholar]
- Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Zhang K, Theall KP, Jiang Y, Bartlett CH, Koehler M, Slamon D. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–439 [CrossRef] [PubMed] [Google Scholar]
- Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, Raber-Durlacher JE, Sonis ST, Elad S. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014;120:1453–1461 [CrossRef] [PubMed] [Google Scholar]
- Altenburg JD, Farag SS. The potential role of PD0332991 (Palbociclib) in the treatment of multiple myeloma. Expert Opin Investig Drugs 2015;24:261–271 [CrossRef] [PubMed] [Google Scholar]
- Schultz KA, Petronio J, Bendel A, Patterson R, Vaughn DJ. PD0332991 (palbociclib) for treatment of pediatric intracranial growing teratoma syndrome. Pediatr Blood Cancer 2015;62:1072–1074 [CrossRef] [PubMed] [Google Scholar]
All Figures
 |
Fig. 1 Appearance of ulcers at the first consultation. |
| In the text | |
 |
Fig. 2 (a) Laser biostimulation, (b) healing of the dorsal side of the tongue at 15 days. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


