| Issue |
J Oral Med Oral Surg
Volume 24, Number 2, June 2018
|
|
|---|---|---|
| Page(s) | 72 - 75 | |
| Section | Cas clinique et revue de la littérature / Up-to date review and case report | |
| DOI | https://doi.org/10.1051/mbcb/2017033 | |
| Published online | 29 June 2018 | |
Up-to Date Review And Case Report
Papulopustular lesions of the face caused by panitumumab: case report and literature review
1
Oral Surgery Department, CHU de Rennes,
2 rue Henri le Guilloux,
35000
Rennes, France
2
Stomatology and Maxillofacial Surgery Department, Centre Hospitalier Départemental de Vendée,
Boulevard Stéphane Moreau,
85000
La Roche-sur-Yon, France
* Correspondence: johnr1988@hotmail.fr.
Received:
31
October
2017
Accepted:
19
December
2017
Introduction: Panitumumab (VECTIBIX®) is a monoclonal antibody used alone or in combination with a chemotherapy for management of metastatic colorectal cancer. Observation: A patient treated with this protocol manifested skin lesions; the etiological diagnosis was difficult. The lesions, namely a papulopustular rash at the lower third of the face, and the medical history allowed to diagnose an acute skin toxicity case due to this monoclonal antibody. Commentary: Many side effects are related to the panitumumab, among which dermatologic adverse events having already been the subject of some publications. Nevertheless, several studies conclude that the therapeutic benefit of this epidermal growth factor receptor inhibitor makes acceptable these complications. Conclusion: Stop treatment and corticosteroids allowed a whole and quick disappearance of skin lesions. Alongside dermatologists and infectious diseases specialists, the opinion of an oral surgeon was useful to provide an answer to these symptoms.
Key words: panitumumab / drug toxicity / acneiform eruption
© The authors, 2018
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Many cancers overexpress the epidermal growth factor receptor (EGFR), which promotes their development and growth. This receptor therefore represents a therapeutic target of choice for oncologists, through the use of certain targeted anticancer therapies. The case presented concerns a complication attributable to such treatment.
Observation
A 64-year-old man was referred by the Infectious Diseases Department for the purpose of establishing an etiological diagnosis for perioral skin lesions that appeared during the fourth course of his chemotherapy for an incidental right metastatic colorectal adenocarcinoma detected 6 months earlier. A hemicolectomy was performed and the patient was treated with FOLFIRI [folinic acid drug combination (LEUCOVORIN®), 5-fluorouracil (ADRUCIL®) and irinotecan (CAMPTOSAR®)], and panitumumab (VECTIBIX®).
With regard to past medical history, the patient had ischemic heart disease (treated by an angioplasty and active stent). He was under treatment with acetylsalicylic acid (KARDEGIC®), ivabradine (PROCORALAN®), and atorvastatin (TAHOR®). He had no known allergies.
Because of superinfected facial dermatitis, the patient had already been given antibiotics for several days in the following order: amoxicillin (CLAMOXIL®), amoxicillin/clavulanic acid (AUGMENTIN®), and clindamycin (DALACINE®). However, no improvement was observed. The patient did not show improvement despite being prescribed an antiviral valacyclovir (ZELITREX®) and an antimycotic fluconazole (TRIFLUCAN®). The bacteriological and virological test results were negative. The blood tests were unremarkable.
In previous courses of treatment, the adverse skin reactions were minimal. Betamethasone (DIPROSONE®) and doxycycline (VIBRAMYCIN®) had been prescribed prophylactically, resulting in only minor lesions, categorized as grades I–II, according to the classification by Common Terminology Criteria for Adverse Events version 4.0 (CTCAEv4) of the National Cancer Institute [1]. These lesions had appeared on the face, trunk, and back.
This time the symptoms were more severe and were strictly confined to the face. An extraoral examination revealed a very painful perioral erythematous plaque, requiring an augmented analgesic treatment including clonazepam (RIVOTRIL®), oxycodone (OXYNORM®), and alprazolam (XANAX®). In addition, there was extensive desquamation with crusting, which proved difficult to remove, and bleeding papules (Fig. 1). There was a slight erythema of the nose. There was no adenopathy. The intraoral examination was strictly normal except for the presence of reflex trismus. The mucous membrane of the vermilion showed moderate superficial inflammation.
There was a correlation between the timing of these symptoms and the treatments the patient received.
Because panitumumab-induced skin reaction was suspected, this treatment was discontinued, and the patient was placed on corticosteroids, i.e., prednisone (CORTANCYL®) and clobetasol propionate (DERMOVAL®). As a result of the change, the lesions cleared up completely and immediately, supporting the diagnosis. Because of this complication, a consumer drug advisory was issued by the manufacturer.
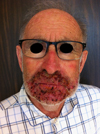 |
Fig. 1 Papulopustular lesions of the face caused by panitumumab: front view of the face. |
Commentary
Every year, one million colorectal cancer cases are diagnosed worldwide, and 50% of the patients develop metastasis. It is the second-most morbid cancer in the world.
There are two chemotherapy protocols for treating metastasis–FOLFOX [folinic acid drug combination (LEUCOVORIN®), 5-fluorouracil (ADRUCIL®) and oxaliplatin (ELOXATINE®)] and FOLFIRI. Certain agents belonging to two families of targeted therapies can be also be combined—a monoclonal antibody inhibiting the vascular endothelial growth factor (VEGF) pathway or two antibodies inhibiting the EGFR pathway.
Panitumumab (VECTIBIX®) belongs to this second family of targeted therapies [2]. According to the European Medicines Agency, it can be used in the first-line treatment (in combination with a FOLFOX or FOLFIRI protocol), in second-line treatment combined with a FOLFIRI protocol, for patients who have received a first-line chemotherapy regimen comprised of fluoropyrimidine (excluding irinotecan), or as a monotherapy (after chemotherapy failure). Its use is subject to the determination of the KRAS mutational status in diagnosing metastatic colorectal cancer [3].
The present case had a resectable adenocarcinoma, classified as pT2N1a with a wild-type KRAS. He had excisional skin surgery followed by adjuvant chemotherapy with FOLFIRI. The tumor’s non-mutated nature allowed it to be combined with panitumumab. In fact, several publications have demonstrated the superiority of the FOLFIRI-panitumumab protocol, compared to a treatment based solely on chemotherapy, in subjects with a wild-type KRAS [4,5].
Cetuximab (ERBITUX®), another anti-EGFR, can be used in a similar fashion to panitumumab. On the other hand, in case of a KRAS mutation-positive tumor, the antiangiogenic anti-VEGF agent bevacizumab (AVASTIN®) is the preferred choice [6,7].
In the present case, the patient presented with a papulopustular rash, which is sometimes referred to as an “acneiform rash”. This cutaneous manifestation, already well-described in the literature, occurs in 60–80% patients receiving anti-EGFR treatment [8,9].
In 2014, Bergman et al. published their follow-up results from a cohort of 32 patients, 85% of which developed a skin rash during the second panitumumab course. Among them, 41% had a mild form, 38% had a moderate form, and 21% a severe form, similar to the clinical situation described for the current case [10].
The anti-EGFR agent cetuximab causes similar lesions, as reported in the case report with two cases published by D’Alessio et al. (2016) [11].
Physiologically, EGFR expresses itself particularly in the skin, where it is essential for the maintenance of cutaneous homeostasis. Its anticancer inhibition results in significant dermatological toxicity [9]. Apart from these acneiform eruptions, anti-EGFR agents may also cause cutaneous mucositis, dry skin, paronychia, hypersensitivity reactions, or hair damage [8]. Oral complications related to anti-EGFR have also been described, but they are less common than the cutaneous complications. These are mainly mucositis (to be differentiated from chemotherapy-induced varieties) and aphthous ulcers [12,13].
More broadly, among the side effects associated with anti-EGFR, there are also infectious complications and episodes of febrile neutropenia. This was confirmed by a meta-analysis involving 28 trials and approximately 15 000 patients [14]. Given the high frequency of skin lesions such as those described in our patient, the field of onco-dermatology has been developing in recent years [15]. For a long time, the lack of clear recommendations, combined with specific criteria for evaluating lesions to facilitate classification, presented a significant obstacle to providing patients with the best possible care [8].
In 2010, the Multinational Association of Supportive Care in Cancer skin toxicity study group proposed a classification scale of skin lesions induced by anti-EGFR treatments according to their clinical appearance [16].
Our patient’s lesions were in grade III–IV. Indeed, the extent of the cutaneous involvement <10%, which is the cutoff for classifying the lesions as grade II. However, the repercussions (eating habits, hygiene, social relations) and symptoms validate such a classification.
Management of such cases will depend on the degree of involvement. Thus, in 2014, Bergmann et al. introduced their codification system for the treatment of these iatrogenic skin lesions, which includes treatment using antibiotics and corticosteroids [10].
From a preventive standpoint, a meta-analysis done in 2015, involving 116 patients, concluded that antibiotic prophylaxis was beneficial in combination with monoclonal antibody therapy [17]. In 2016, a similar publication by Petrelli et al., this time grouping 13 studies and 1073 patients, also favored such a protocol. In fact, tetracycline usage in the weeks preceding the treatment made it possible to decrease the incidence and the severity of the cutaneous reactions [18]. Recently, skin desensitization, and innovative technique, have been reported to significantly decrease symptoms in two patients [11].
In 2011, Bennett et al. conducted a study on the quality of life of patients receiving such treatments. According to them, panitumumab usage coupled with chemotherapy significantly increases the patient’s survival without cancer progression or a compromised quality of life [19]. Similarly, Koukakis et al. concluded in 2016 reported that there were no significant differences between the quality of life reported by patients who received this monoclonal antibody and that reported by patients who did not receive it [20].
For most patients, it is possible to predict their response to the anticancer treatment by the magnitude of their skin reaction. In fact, Jaka et al. found that all the patients whose tumors healed completely also had skin reactions [17].
With regard to this patient, after a right hepatectomy, which had been scheduled because of his metastasis, he also received what is referred to as “closure” chemotherapy via a single FOLFIRI protocol. Subsequent imaging studies showed no signs of relapse.
Conclusion
The indications for targeted therapies are increasing, along with the consequent increase in incidence of associated complications. Oral surgeons, along with dermatologists and infectious disease specialists, are responsible for managing such lesions. Oral surgeons must therefore be involved in the multidisciplinary consultation during which treatment decisions will be made. This will allow for monitoring patients adequately as well as any treatment-related complications that may occur.
Conflicts of interests
The authors declare that they have no conflicts of interest in relation to this article
References
- Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, Lacouture ME. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol 2012;67:1025–1039. [CrossRef] [PubMed] [Google Scholar]
- Keating GM. Panitumumab: a review of its use in metastatic colorectal cancer. Drugs 2010;70:1059–1078. [CrossRef] [PubMed] [Google Scholar]
- Cartwright TH. Treatment decisions after diagnosis of metastatic colorectal cancer. Clin Colorectal Cancer 2012;11:155–166. [CrossRef] [PubMed] [Google Scholar]
- Karthaus M, Hofheinz R-D, Mineur L, Letocha H, Greil R, Thaler J, et al. Impact of tumour RAS/BRAF status in a first-line study of panitumumab + FOLFIRI in patients with metastatic colorectal cancer. Br J Cancer 2016;115:1215–1222. [CrossRef] [PubMed] [Google Scholar]
- Peeters M, Oliner KS, Price TJ, Cervantes A, Sobrero AF, Ducreux M. Analysis of KRAS/NRAS mutations in a phase III study of panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal cancer. Clin Cancer Res 2015;21:5469–5479. [CrossRef] [PubMed] [Google Scholar]
- McLean J, Rho YS, Kuruba G, Mamo A, Gilabert M, Kavan T, Panasci L, Melnychuk D, Batist G, Kavan P. Clinical practice patterns in chemotherapeutic treatment regimens for metastatic colorectal cancer. Clin Colorectal Cancer 2016;15:135–140. [CrossRef] [PubMed] [Google Scholar]
- Cremolini C, Schirripa M, Antoniotti C, Moretto R, Salvatore L, Masi G, Falcone A, Loupakis F. First-line chemotherapy for mCRC—a review and evidence-based algorithm. Nat Rev Clin Oncol 2015;12:607–19. [CrossRef] [Google Scholar]
- Agero ALC, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 2006;55:657–670. [CrossRef] [PubMed] [Google Scholar]
- Sibaud V, Delord J-P, Kidney C, Gangloff D, Garrido-Stowhas I. Dermatological toxicity of new targeted anti-cancer therapies used in oncodermatology. Ann Chir Plast Esthét. 2012;57:106–113. [CrossRef] [Google Scholar]
- Bergman H, Walton T, Del Bel R, Seki JT, Rafii A, Xu W, Koren G, Shear N, Kryzanawska MK, Howell D, Liu G. Managing skin toxicities related to panitumumab. J Am Acad Dermatol 2014;71:754–759. [CrossRef] [PubMed] [Google Scholar]
- D’Alessio A, Cecchini S, Di Mauro D, Geroli L, Villa S, Quadri A, Resta D, Fortugno C. Cutaneous toxicity from epidermal growth factor receptor inhibitors: would a subcutaneous desensitization be helpful? Case report. Tumori J. 2016;102(Suppl. 2):65–68. [CrossRef] [Google Scholar]
- Sibaud V, Boralevi F, Vigarios E, Fricain J-C. Endobuccal toxicity of targeted anti-cancer therapies. Ann Dermatol Venereology 2014;141:354–363. [CrossRef] [PubMed] [Google Scholar]
- Sibaud V, Vigarios E. Oral toxicities of targeted anti-cancer therapies. Oral Medicine Chir Oral 2015;21:149–155. [Google Scholar]
- Funakoshi T, Suzuki M, Tamura K. Infectious complications in cancer patients treated with anti-EGFR monoclonal antibodies cetuximab and panitumumab: A systematic review and meta-analysis. Cancer Treat Rev 2014;40:1221–1229. [CrossRef] [PubMed] [Google Scholar]
- Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: The study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol 2011;65:624–635. [CrossRef] [PubMed] [Google Scholar]
- Lacouture ME, Maitland ML, Segaert S, Setser A, Baran R, Fox LP, Epstein JB, Barasch A, Einhorn L, Wagner L, West DP, Rapoport BL, Krsi MG, Basch E, Eaby B, Kurtin S, Olsen EA, Chen A, Dancey JE, Trotti A. A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group. Support Care Cancer 2010;18:509–522. [CrossRef] [PubMed] [Google Scholar]
- Jaka A, Gutiérrez-Rivera A, López-Pestaña A, del Alcázar E, Zubizarreta J, Vildosola S, Arrequi MA, Sarasqueta C, Lobo C, Tuneu A. Predictors of tumor response to cetuximab and panitumumab in 116 patients and a review of approaches to managing skin toxicity. Actas Dermo-Sifiliográficas Engl Ed. 2015;106:483–492. [CrossRef] [Google Scholar]
- Petrelli F, Borgonovo K, Cabiddu M, Coinu A, Ghilardi M, Lonati V, Barni S. Antibiotic prophylaxis for skin toxicity induced by antiepidermal growth factor receptor agents: a systematic review and meta-analysis. Br J Dermatol 2016;175:1166–1174. [CrossRef] [PubMed] [Google Scholar]
- Bennett L, Zhao Z, Barber B, Zhou X, Peeters M, Zhang J, Xu F, Weiezorek J, Douillard JY. Health-related quality of life in patients with metastatic colorectal cancer treated with panitumumab in first- or second-line treatment. Br J Cancer 2011;105:1495–1502. [CrossRef] [PubMed] [Google Scholar]
- Koukakis R, Gatta F, Hechmati G, Siena S. Skin toxicity and quality of life during treatment with panitumumab for RAS wild-type metastatic colorectal carcinoma: results from three randomised clinical trials. Qual Life Res 2016;25:2645–2656. [CrossRef] [PubMed] [Google Scholar]
All Figures
 |
Fig. 1 Papulopustular lesions of the face caused by panitumumab: front view of the face. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


