| Issue |
J Oral Med Oral Surg
Volume 30, Number 4, 2024
|
|
|---|---|---|
| Article Number | 25 | |
| Number of page(s) | 10 | |
| DOI | https://doi.org/10.1051/mbcb/2024031 | |
| Published online | 02 December 2024 | |
Systematic Review
What is the effectiveness of chitosan in promoting the healing of tooth extraction sockets? A systematic review
Al-Fallujah Specialized Dental Center, Al-Anbar Health Directorate, Fallujah, Anbar, Iraq
* Correspondence: asawerafayyad@gmail.com
Received:
18
September
2024
Accepted:
23
October
2024
Purpose: This review aimed to determine the efficacy of chitosan in hemostasis and wound healing and its effectiveness in reducing pain and inflammation in the extraction socket compared to the control group without chitosan. Methods: This review was performed through an electronic data search in PubMed, ScienceDirect, Google Scholar, Cochrane Library, and Lilacs. A total of 4 randomized clinical trial studies that fulfilled the inclusion criteria were included in the review. Results: All evaluated studies showed that chitosan significantly improved wound healing of extraction sockets and lead to significant acceleration in the time of hemostasis (p >.001) (p = 0.0278). 70% of evaluated studies found that chitosan significantly reduced postoperative pain (p = 0.0001) (p = 0.002), and 65% showed that chitosan reduced postoperative inflammation. Conclusion: According to the evaluated studies, chitosan was effective in promoting socket healing, as there was a significant difference between the chitosan group and the control group, with substantial improvement in wound healing and hemostasis.
Key words: Tooth socket / extraction socket / healing / chitosan
© The authors, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The extraction of teeth is a standard procedure encountered in dental clinics, and one of the essential measures after extraction is the control of bleeding and the achievement of hemostasis. However, continuous bleeding after extraction is a common medical complication, especially in patients with antithrombosis [1].
Various measurements can be taken to reduce bleeding from the socket, and hemostatic agents are one available method [2]. Recently, it has been focused on using biological materials like collagen, chitin, and their derivatives, which can accelerate the healing process at cellular and molecular levels [3].
Chitosan (CS) is a biocompatible, biodegradable, natural, non-toxic polysaccharide and has demonstrated antibacterial properties [4]. The biodegradable properties lead to the formation of non-toxic oligosaccharides of varying lengths that can be metabolized through different pathways and subsequently excreted from the body [5]. The antimicrobial properties of chitosan can be attributed to two mechanisms: (a) its binding to DNA via protonated amino groups and (b) its interaction with the bacterial cell membrane that bears a negative charge [6].
It has a positive charge, which attracts negatively charged red blood cells and platelets; therefore, through this way, it achieves a robust seal in surgical wounds. It achieves hemostasis, aids in healing, serves as a scaffold for tissue engineering, and functions as a system for drug delivery. Furthermore, chitosan has a hydrophilic surface that promotes the cellular component's proliferation, adhesion, and differentiation [7].
It is available in various forms, including sponge, film, fibers, and hydrogels. These forms are versatile biomaterials; they act as two or three-dimensional scaffolds in the wound dressing [8]. Also, HemCon® dental dressing is one of the available commercial chitosan products that can control bleeding [9].
Recently, various systematic studies have attempted to gather the existing evidence about the application of chitosan [10,11]. Nevertheless, their focus was not merely on socket healing; instead, they broadened their study to involve a more comprehensive array of indications and various levels of dentistry. Consequently, this systematic review aimed to evaluate the effect of chitosan in socket healing to address the following specific questions: in patients with fresh sockets, what is the efficacy of chitosan in promoting hemostasis and wound healing and in reducing pain and inflammation in the extraction socket compared to the control group without chitosan?
Materials and methods
Study design
This systematic review was designed using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement and the guidelines of known literature to write the systematic review [12,13].
The central questions that fulfilled the acronym PICO were [14]:
P = Patients with extracted tooth sockets.
I = Socket healing using chitosan.
C = Compared to socket healing without chitosan using conventional measures and other interventions.
O = The presence of one or more outcomes: time to achieve hemostasis, wound healing, pain, and inflammation.
Search strategy
The search was conducted in June 2024 without any limitations on the time of publication, and the significant databases used are (PubMed, ScienceDirect, Google Scholar, Cochrane Library, and LILACS). The keywords used to conduct the research are (“Socket healing” OR “Tooth extraction socket” OR “Tooth extraction” OR “Socket preservation” OR “Ridge preservation” AND “chitosan”).
Inclusion criteria
Randomized clinical trials (RCTs) that evaluate the use of chitosan in promoting socket healing after tooth extraction.
Studies include the treatment of the fresh socket.
Studies written in the English language.
Human studies.
Full-text articles.
Exclusion criteria
Studies written in a language other than English language
Studies that are randomized clinical trials.
Studies not related to the role of chitosan in socket healing.
Review articles, case reports, case series, book chapters.
In vivo, in vitro, and animal studies.
Risk of bias assessment
The risk of bias in RCTs was assessed according to the Cochrane Guidelines using the RoB2 tool about the following domains (bias created by the randomization process, bias caused by deviations from intended interventions, bias because of missing outcome data, bias in measurement of the outcome, and bias selection of the reported result). After that, each study was assessed as low risk, some concern, and a high risk of bias.
Data extraction
The data extraction was arranged in an organized sheet involving the design of the study, country, mean age, number of patients, medical condition, surgical procedure, and region of extraction. The following parameters were established for data analysis:
Primary Outcomes: Time to achieve hemostasis and wound healing.
Secondary Outcomes: Pain and inflammation.
Results
Study inclusion
A total of 5673 studies were collected from databases (PubMed, ScienceDirect, Google Scholar, Cochrane Library, and LILACS). After removing the duplicate studies, which were 1712, 3961 titles and abstract records were screened, from which 3921 were excluded, and 40 articles were assessed for eligibility, of which 36 articles were excluded according to exclusion criteria, and 4 articles were included in the review (Fig. 1).
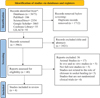 |
Fig. 1 Prisma flow diagram. |
Study design
In this review, four studies were analyzed. All of them were randomized clinical trials (RCTs); they included two intervention groups: a test group that used chitosan and a control group that used conventional measures and other interventions.
Two studies measured the effectiveness of chitosan only compared to placebo interventions, and two other studies compared chitosan in combination with different materials to placebo interventions. A total of 157 patients were included in this review, and the average age across studies was 18 to 86 years. Two studies reported the tooth socket to which the interventions were applied, which is the mandibular third molar [15,16], and two studies didn't report the socket site for intervention application [17,18] (Tab. I).
Study Design.
Risk of bias assessment
The risk of bias in three studies was judged to be some concern [16–18], of which two studies [16,18] were assessed with some concerns, as they have a low risk of bias in the randomization process, deviations from the intended interventions, missing outcome data, and measurement of outcome domains, and have assessed some concern in the selection of the reported result domain. The study of Radhakrishna [17] evaluated as some concern, as it has some concerns in the randomization process and selection of the reported result domains and assessed them as low risk of bias in deviations from the intended interventions, missing outcome data, and measurement of outcome domains. The study of Sáez-Alcaide (2020) [15] was assessed to have a high risk of bias; it has a low risk of bias in the randomization process, deviations from the intended interventions, and measurement of outcome domains but has a high risk of bias in missing outcome data domain and has some concern in the selection of the reported result domain (Fig. 2).
 |
Fig. 2 Risk of bias assessment of RCTs studies using cochrane tool. |
Primary outcomes
Time to achieve hemostasis
Two studies measured the time to achieve hemostasis in the extraction socket through a socket treatment with chitosan dental dressing compared to a cotton pressure pack in the Radhakrishna et al. study [17], and the treatment of socket using HemCon® Dental Dressing (HDD) compared to hemostatic sponge (CollaPlug, Zimmer Dental) in the Pippi et al. study [18]. In the study of Radhakrishna et al. [17], compared to the conventional pressure pack used in the control group, the chitosan dressing used in the test group was more effective in achieving hemostasis; it achieved hemostasis in a shorter time with a statistically significant difference (p < .001). Pippi et al. [18] also found a significant difference between HDD in the test group and the hemostatic sponge in the control group in the time of achieving hemostasis (p = 0.0278); the bleeding time was lower in the socket-treated with the hemostatic sponge than in the socket treated with HDD (Tab. II).
Time to achieve hemostasis outcomes in evaluated studies.
Wound healing
All four studies evaluated wound healing using different measures: the alveolar clinical healing index, the Madrazo-Jimenez [19] modified scale, and the percentage of healing. All studies showed statistically significant differences between the test group and the control group; Radhakrishna et al. [17] evaluated wound healing after 1 week and demonstrated that the test group showed higher levels of healing than the control group. Lope-Lopez et al. [16] compared healing between the two groups to evaluate wound healing and found that the Bexident Post (BP) group achieved better healing than the bicarbonate (BC) group (p = 0.0001). Sáez-Alcaide et al. [15] evaluated wound healing on days 2 and 7 and showed that wound healing was improved more in the test group than in the control group. Pippi et al. [18] evaluated wound healing at the time of suture removal and found that wound healing was better in the test group than in the control group (Tab. III).
Wound healing assessment outcomes in evaluated studies.
Secondary outcomes
Pain
Three studies evaluated postoperative pain using different pain scales. Lope-Lopez et al. [16] assessed the patient's postoperative pain by comparing socket treatment with BP gel in the test group to socket treatment with BC oral rinse in the control group, by using Visual Analogue Scale (VAS) through the patient following six hours of the procedure and at about 11 am every day for seven days after the procedure. In the first six hours after the procedure, the two groups had no difference in VAS pain score (VAS = 6.5). However, on the third and seventh days, there was a significant difference between the test and control groups, as there was a substantial reduction in the VAS score in the test group (3.7 vs. 5.3) (p = 0.0001). Sáez-Alcaide et al. [15] also evaluated postoperative pain for seven days using the VAS score in a comparison between socket treatment with topical gel composed of chitosan, 0.2% chlorhexidine, allantoin, and dexpanthenol in the test group and socket treatment with a placebo gel, which includes all the components from the commercially available gel except chitosan, 0.2% chlorhexidine, allantoin and dexpanthenol in the control group, there was a significant difference between the two groups, he found a substantial reduction in the pain score in the test group (p = 0.002). Pippi et al. [18] evaluated postoperative pain in the evening of surgery (PS T1) and the next morning by the phone (PS T2) and 5 ±1 days after surgery during the suture removal using a 0-10 subjective scale (pain score), in a comparison between the test group in which the socket treated with HDD and the control group in which the socket treated with a hemostatic sponge. He showed that PS T1 was lower in the control group than in the test group, but this difference wasn't significant (p = 0.7263); PS T2 was lower in the test group than in the control group (p = 0.3843). However, there was a substantial difference in the PS T3 (p = 0.0481), as the test group showed substantially lower pain scores than the control group (Tab. IV).
Pain assessment in evaluated studies.
Assessment of inflammation in evaluated studies.
Inflammation
Three studies evaluated inflammation in the extraction socket; one showed no significant difference between the test group in which the socket is treated by chitosan dental dressing and the control group in which the socket is treated with a cotton pressure pack [17]. Two other studies showed a significant difference between test and control groups in inflammation. Lope-Lopez et al. [15] found a substantial difference between the BP group and the BC group; BP gel reduced inflammation by about 50% more than BC rinse (p = 0.001). Sáez-Alcaide et al. [15] showed inflammation outcomes in many measures. They compared the presence of alveolitis between the test and control groups and found no significant difference between the two groups (p = 0.063). They also evaluated swelling in three parameters (Go-Eye, Tg-Com, Tg-Pg), and they saw the swelling in all three measurements reduced in the test group more than in the control group with a high significant difference (Go-Eye p = 0.009, Tg-Com p <0.001, Tg-Pg p <0.001). They also compared mouth-opening between the test and control, and they found significant differences between them (p = 0.005); the test group showed lower values of trismus than the control group (Tab. V).
Discussion
This review was performed to assess the effectiveness of chitosan on tooth extraction socket healing, including assessment of time to achieve hemostasis, wound healing, inflammation, and postoperative pain. It is limited to RCT studies.
In recent years, advancements in the scientific and biomedical fields have facilitated the development of new biomaterials that are progressively more effective and secure. Significant progress has been achieved in the research of marine-based biomaterials [20–22]. Presently, marine-derived biomaterials are effectively utilized in surgical, orthopedic, reconstructive plastic surgery, aesthetic procedures, and dentistry applications [23,24].
CS is one of these materials utilized throughout various dental disciplines and has multiple functions. It is a polysaccharide derived from plentiful natural sources and possesses significant potential as a biomaterial for tissue regeneration. It is derived from the deacetylation of chitin, a polymer found in the exoskeletons of crustaceans, mollusks, and insects, as well as in the cell walls of the fungus [4].
Its chemical structure has three reactive functional groups: one amine (NH2) and two hydroxyl groups (OH). The amine groups impart a cationic characteristic to CS, which enhances its affinity for anionic biomolecules, including sialic acid, sulfonic acid, and glycosaminoglycans (GAGs) prevalent in mucous secretions and the extracellular matrix [25,26].
Indeed, CS is regarded as the only natural polysaccharide possessing this feature [27]. Through ionic interactions, chitosan can adhere to mucous tissue, a property known as mucoadhesiveness [28,29].
This feature is significant for tissue engineering, as a scaffold that interacts with glycosaminoglycans (GAGs) and tissue proteoglycans may enhance the integration of cytokines and tissue growth factors, given that many of these substances have an affinity for GAGs [30,31]. This was proven in this review of primary outcomes in which the CS achieved faster hemostasis and improved wound healing than the control group (Tabs. II and III). The positive charge of chitosan makes it able to attract negative charge blood vessels and platelets to achieve hemostasis [7]. Several systematic reviews prove chitosan can fasten hemostasis and improve wound healing [32,33]. Shen et al. have shown that chitosan exposure increases the release of growth factors from human platelets, which elucidates our favorable outcomes [34].
In addition, several in vitro studies have demonstrated that chitosan has immunomodulatory features and encourages the release of IL-1 from macrophages. This, in turn, augments fibroblast proliferation and collagen production. When chitosan was applied, wounds demonstrated a rise in osteopontin and collagen and a significant infiltration of polymorphonuclear (PMN) leukocytes. Since chitosan has a higher degree of deacetylation than chitin, it appears to have more active fibroblasts. It is more resistant to bursting wounds, which could assist in explaining our results that chitosan promotes wound healing (Tab. III) [35].
Furthermore, it enhances wound healing by stimulating bone formation and inhibiting the growth of microorganisms such as Candida, Klebsiella, Streptococci, Staphylococci, and Pseudomonas [36]. It is also associated with antibacterial properties, as examined in in vitro studies. The results indicated that chitosan increased the permeability of both the inner and outer membranes, ultimately disrupting bacterial cell membranes and releasing their contents. Consequently, HDD functions as an antibacterial barrier against several Gram-positive and Gram-negative microbes, including Acinetobacter baumannii, vancomycin-resistant enterococcus (VRE), and methicillin-resistant Staphylococcus aureus (MRSA) [37,38].
Numerous studies have demonstrated the considerable influence of chitosan on bone repair and regeneration processes as the biodegradability and biocompatibility of chitosan facilitate its application in hard tissue healing. The process functions on the principle of offering a temporary scaffold, enabling the progressive dissolution of the implant, which is subsequently replaced by natural bone tissue. This confers a significant degree of stability upon chitosan, establishing a foundation for forming new bone cells. Chitosan in sponge form has been shown in specific tests to stimulate osteoblasts, perhaps facilitating osteogenesis [39].
A study was performed to examine the impact of chitosan on the healing of dental sockets post-tooth extraction, specifically regarding its viability as a biomaterial for bone regeneration. The outcomes after 10 weeks were compelling. The central and apical regions of the treated sockets exhibited markedly enhanced bone density. The results indicate that bone tissue regeneration occurs more rapidly in chitosan-filled dental sockets than in untreated ones [40].
This review clarified that there is a significant reduction in inflammation in the chitosan group (Tab. V). In vivo and in vitro studies have demonstrated that chitosan has a pro-inflammatory effect. When chitosan oligomers undergo enzymatic degradation in the wound environment, they stimulate macrophages and significantly enhance their migratory activity [41].
In addition, many studies show that diverse chitosan-based biomaterials have anti-inflammatory effects such as downregulating interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and PGE2. Chitosan also diminishes the phosphorylation of c-Jun N-terminal kinase (JNK), phosphatidylinositol 3-kinase (PI3K), Protein kinase B (AKB), and nuclear factor kappa B (NF-κB). Chitosan, however, reduces the activities of matrix metalloproteinase 1 (MMP-1), matrix metalloproteinase 2 (MMP-2), caspase 3 (case-3), and caspase 9 (casp-9), resulting in anti-apoptotic qualities of chitosan [42].
A significant reduction in postoperative pain in extraction sockets treated with chitosan has been demonstrated in this review (Tab. IV). This observation may be explained by the ability of chitosan to produce residual acetic acid that aids in pain reduction [43].
Other studies also showed the efficacy of chitosan in pain reduction [11,44–46]. Zúñiga-López showed in their research on the effectiveness of chitosan on oral mucositis (OM) that chitosan, when applied, reduces the pain of oral ulcers [11].
Conclusion
This review aimed to offer clinical evidence on the effectiveness of chitosan in treating extraction sockets compared to extraction socket treatment with other interventions. Despite the limitations in data collection, chitosan was shown to promote socket healing effectively in achieving hemostasis faster, reducing postoperative pain, improving and accelerating wound healing, and reducing inflammation.
Funding
This paper didn't obtain any funding.
Conflicts on intesrest
The author declares to have no conflict of interest.
Data availability statement
Data will be available upon reasonable request from the corresponding author.
References
- Aframian DJ, Lalla RV, Peterson DE. Management of dental patients taking common hemostasis-altering medications. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2007;103:S45.e1– S45.e11. [Google Scholar]
- Sammartino G, Ehrenfest DMD, Carile F, Tia M, Bucci P. Prevention of hemorrhagic complications after dental extractions into open heart surgery patients under anticoagulant therapy: the use of leukocyte- and platelet-rich fibrin. J Oral Implantol. 2011;37:681–690. [CrossRef] [PubMed] [Google Scholar]
- Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29:322–337. [CrossRef] [Google Scholar]
- Qasim S, Zafar M, Najeeb S, Khurshid Z, Shah A, Husain S, et al. Electrospinning of chitosan-based solutions for tissue engineering and regenerative medicine. Int J Mol Sci. 2018;19:407. [CrossRef] [Google Scholar]
- Pusateri AE, McCarthy SJ, Gregory KW, Harris RA, Cardenas L, McManus AT, et al. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J Trauma: Injury, Infection, Critical Care 2003;54:177–182. [CrossRef] [PubMed] [Google Scholar]
- Pogorielov MV, Sikora VZ. Chitosanas a hemostatic agent: current state. Eur J Med Ser B. 2015;2:24–33. [CrossRef] [Google Scholar]
- Eldibany RM. Platelet rich fibrin versus Hemcon dental dressing following dental extraction in patients under anticoagulant therapy. Tanta Dent J 2014;11:75–84. [CrossRef] [Google Scholar]
- Oryan A, Sahvieh S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int J Biol Macromol 2017;104:1003–1011. [CrossRef] [PubMed] [Google Scholar]
- Gupta A, Rattan V, Rai S. Efficacy of Chitosan in promoting wound healing in extraction socket: a prospective study. J Oral Biol Craniofac Res 2019;9:91–95. [CrossRef] [PubMed] [Google Scholar]
- Cicciù M, Fiorillo L, Cervino G. Chitosan use in dentistry: a systematic review of recent clinical studies. Mar Drugs 2019;17:417. [CrossRef] [PubMed] [Google Scholar]
- Zúñiga-López CM, Márquez-Pérez K, Argueta-Figueroa L, Bautista-Hernández MA, Torres-Rosas R. Chitosan for the treatment of inflammation of the oral mucosa: a systematic review. Med Oral Patol Oral Cir Bucal 2024; e9–e 17. [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647–g7647. [CrossRef] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021 Mar 29;n 71. [Google Scholar]
- Miller SA, Forrest JL. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J Evid Based Dent Pract 2001;1:aed0010136. [CrossRef] [Google Scholar]
- Sáez-Alcaide LM, Molinero-Mourelle P, González-Serrano J, Rubio-Alonso L, Bornstein MM, López-Quiles J. Efficacy of a topical gel containing chitosan, chlorhexidine, allantoin and dexpanthenol for pain and inflammation control after third molar surgery: A randomized and placebo-controlled clinical trial. Med Oral Patol Oral Cir Bucal 2020;e644–e651. [PubMed] [Google Scholar]
- Lope-Lopez J, Jan-Pallí E, González-Navarro B, Jané-Salas E, Estrugo-Devesa A, Milani M. Efficacy of chlorhexidine, dexpanthenol, allantoin and chitosan gel in comparison with bicarbonate oral rinse in controlling post-interventional inflammation, pain and cicatrization in subjects undergoing dental surgery. Curr Med Res Opin 2015;31:2179–2183. [CrossRef] [PubMed] [Google Scholar]
- Radhakrishna S, Shukla V, Shetty SK. Is Chitosan dental dressing better than cotton gauze in achieving hemostasis in patients on antithrombotics? J Oral Maxillofac Surg 2023;81:224–231. [CrossRef] [PubMed] [Google Scholar]
- Pippi R, Santoro M, Cafolla A. The use of a chitosan-derived hemostatic agent for postextraction bleeding control in patients on antiplatelet treatment. J Oral Maxillofac Surg 2017;75:1118–1123. [CrossRef] [PubMed] [Google Scholar]
- Madrazo-Jimenez M, Rodriguez-Caballero A, Serrera-Figallo M, Garrido-Serrano R, Gutierrez-Corrales A, Gutierrez-Perez J, et al. The effects of a topical gel containing chitosan, 0,2% chlorhexidine, allantoin and despanthenol on the wound healing process subsequent to impacted lower third molar extraction. Med Oral Patol Oral Cir Bucal 2016;0–0. [PubMed] [Google Scholar]
- Ghosh B, Urban MW. Self-repairing oxetane-substituted chitosan polyurethane networks. Science (1979). 2009;323:1458–1460. [CrossRef] [PubMed] [Google Scholar]
- Ortiz C, Boyce MC. Bioinspired structural materials. Science (1979). 2008;319:1053–1054. [CrossRef] [PubMed] [Google Scholar]
- Bonderer LJ, Studart AR, Gauckler LJ. Bioinspired design and assembly of platelet reinforced polymer films. Science (1979). 2008;319:1069–1073. [Google Scholar]
- Jones N. Food: a taste of things to come? Nature 2010;468:752–753. [CrossRef] [PubMed] [Google Scholar]
- Ladet S, David L, Domard A. Multi-membrane hydrogels. Nature 2008;452:76–79. [CrossRef] [PubMed] [Google Scholar]
- Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol 2010;56:290–299. [CrossRef] [PubMed] [Google Scholar]
- Muzzarelli RAA. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr Polym. 2009;76:167–182. [Google Scholar]
- Bellich B, D'Agostino I, Semeraro S, Gamini A, Cesàro A. “The Good, the Bad and the Ugly” of Chitosans. Mar Drugs 2016;14:99. [CrossRef] [PubMed] [Google Scholar]
- Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 2008;26:1–21. [CrossRef] [PubMed] [Google Scholar]
- Sogias IA, Williams AC, Khutoryanskiy VV. Why is Chitosan Mucoadhesive? Biomacromolecules 2008;9:1837–1842. [CrossRef] [PubMed] [Google Scholar]
- Madihally SV, Matthew HWT. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999;20:1133–1142. [CrossRef] [PubMed] [Google Scholar]
- Zeinali R, Biazar E, Keshel SH, Tavirani MR, Asadipour K. Regeneration of full-thickness skin defects using umbilical cord blood stem cells loaded into modified porous scaffolds. ASAIO.J. 2014;60:106–114. [CrossRef] [PubMed] [Google Scholar]
- Agrawal A, Reche A, Agrawal S, Paul P. Applications of chitosan nanoparticles in dentistry: a review. Cureus 2023;4;15(12):e49934. [PubMed] [Google Scholar]
- Minervini G, Franco R, Marrapodi MM, Di Blasio M, Cicciù M, Ronsivalle V. The effectiveness of chitosan as a hemostatic in dentistry in patients with antiplatelet/anticoagulant therapy: systematic review with meta-analysis. BMC Oral Health 2024;24:70. [CrossRef] [PubMed] [Google Scholar]
- Shen E, Chou T, Gau C, Tu H, Chen Y, Fu E. Releasing growth factors from activated human platelets after chitosan stimulation: a possible bio‐material for platelet‐rich plasma preparation. Clin Oral Implants Res 2006;17:572–578. [CrossRef] [PubMed] [Google Scholar]
- Tavaria FK, Costa EM, Pina-Vaz I, Carvalho MF, Pintado MM. A quitosana como biomaterial odontológico: estado da arte. Revista Brasileira de Engenharia Biomédica 2013;29:110–120. [CrossRef] [Google Scholar]
- Gilbert Triplett R, Budinskaya O. New frontiers in biomaterials. Oral Maxillofac Surg Clin North Am 2017;29:105–115. [CrossRef] [PubMed] [Google Scholar]
- Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, P Castano A, Hamblin MR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 2006;27:4157–4164. [CrossRef] [PubMed] [Google Scholar]
- Spagnuolo G, Annunziata M, Rengo S. Cytotoxicity and oxidative stress caused by dental adhesive systems cured with halogen and LED lights. Clin Oral Investig 2004;8:81–5. [CrossRef] [PubMed] [Google Scholar]
- Jeong Park Y, Moo Lee Y, Nae Park S, Yoon Sheen S, Pyoung Chung C, Lee SJ. Platelet derived growth factor releasing chitosan sponge for periodontal bone regeneration. Biomaterials 2000;21:153–159. [CrossRef] [PubMed] [Google Scholar]
- Park J, Choi S, Moon I, Cho K, Chai J, Kim C. Eight‐week histological analysis on the effect of chitosan on surgically created one‐wall intrabony defects in beagle dogs. J Clin Periodontol 2003;30:443–453. [CrossRef] [PubMed] [Google Scholar]
- Chang HH, Wang YL, Chiang YC, Chen YL, Chuang YH, Tsai SJ, et al. A novel chitosan-γPGA polyelectrolyte complex hydrogel promotes early new bone formation in the alveolar socket following tooth extraction. PLoS One 2014;9:e92362. [CrossRef] [PubMed] [Google Scholar]
- Bilginaylar K, Aykac A, Sayiner S, Özkayalar H, Şehirli AÖ. Evaluation of the antiapoptotic and anti-inflammatory properties of chitosan in methotrexate-induced oral mucositis in rats. Mol Biol Rep 2022;49:3237–3245. [CrossRef] [PubMed] [Google Scholar]
- Pogorielov MV, Sikora VZ. Chitosan as a hemostatic agent: current state. Eur J Med Ser B. 2015;2:24–33. [CrossRef] [Google Scholar]
- Atai Z, Atai M, Amini J, Salehi N. In vivo study of antifungal effects of low-molecular-weight chitosan against Candida albicans. J Oral Sci 2017;59:425–430. [CrossRef] [PubMed] [Google Scholar]
- Rahmani F, Moghadamnia AA, Kazemi S, Shirzad A, Motallebnejad M. Effect of 0.5% Chitosan mouthwash on recurrent aphthous stomatitis: a randomized double-blind crossover clinical trial. Electron Physician 2018;10:6912–6919. [CrossRef] [PubMed] [Google Scholar]
- Shao Y, Zhou H. Clinical evaluation of an oral mucoadhesive film containing chitosan for the treatment of recurrent aphthous stomatitis: a randomized, double-blind study. J Dermatol Treat 2020;31:739–743. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Asawer Ahmed Fayyad, 2024. What is the effectiveness of chitosan in promoting the healing of tooth extraction sockets? A systematic review. J Oral Med Oral Surg. 30, 25. https://doi.org/10.1051/mbcb/2024031
All Tables
All Figures
 |
Fig. 1 Prisma flow diagram. |
| In the text | |
 |
Fig. 2 Risk of bias assessment of RCTs studies using cochrane tool. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


