| Issue |
J Oral Med Oral Surg
Volume 24, Number 2, June 2018
|
|
|---|---|---|
| Page(s) | 81 - 88 | |
| Section | Cas clinique et revue de la littérature / Up-to date review and case report | |
| DOI | https://doi.org/10.1051/mbcb/2017040 | |
| Published online | 29 June 2018 | |
Up-to Date Review and Case Report
Benign myoepithelioma of the hard palate: a clinical and histological diagnostic challenge. Case report and literature review
1
Bretonneau Hospital, Department of Oral Surgery,
Paris, France
2
Faculty of Dental Surgery, Paris Descartes University,
Sorbonne Paris Cité, France
3
Department of Oral & ENT Pathology, Pitié-Salpêtrière Hospital, Department of Dermatology, Cochin-Port Royal Hospital,
Paris, France
* Correspondence: louismaffiberthier@laposte.net
Received:
12
September
2017
Accepted:
11
December
2017
Introduction: Myoepithelioma (ME) is a rare salivary gland tumor. Constructed aroung a clinical case, this article aims to gather up up-to-date epidemiological, clinical and histological data about myoeptihelioma with emphasis on the diagnostic approach and differential diagnoses, paraclinical exams and the main histological features reported for its characterization. Observation: A 41-year-old female, presenting a 1-year slowly enlarging palatine nodule was referred to the Oral Pathology Consultation. Clinical data and paraclinic examination were non-specific. A thorough histological examination, comparing clinical data with cyto-architectural and immunostaining profile of the tumor allowed a positive diagnosis of ME. Discussion: The clinical aspect of ME is close from other more frequent tumors within the same areas. Accordingly, its discovery is often incidental and its diagnosis histological. ME display variable architecture and composition, requiring full tumor examination for proper diagnosis. When benign, ME act as mixed tumor regarding local extension, prognosis and recurrence. Malignant ME behaves as a low-grade malignant tumor with metastatic potential. Conclusion: Despite its rarity, ME should be hypothesized in front of a palatine nodule. Clinician and pathologist should be particularly cautious regarding nature, malignancy and follow-up of this tumor, since few data are up-to-now available.
Key words: pathology / oral / myoepithelioma / palate / neoplasms
© The authors, 2018
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Palate is one of the site that are the most affected by tumoral processes in the oral cavity. Among them, minor salivary gland tumors present with a great polymorphism, and a high proportion of malignancies. Diagnosing a palatine nodule remains a challenge for the clinician since many distinct entities present with the same clinical features, but display different prognoses, local aggressivity, metastatic potential, and treatment strategies.
Among those tumors, Myoepithelioma is a rare benign salivary gland neoplasm, composed of ectoderm-derived contractile cells that act as smooth muscle cells, called myoepithelial cells. This tumor was firstly described in 1943 by Sheldon et al. and 50 years later, considered a distinct entity among salivary gland adenomas and no longer as a variant of pleomorphic adenoma by WHO [1].
Considering that approximately half of the minor salivary gland prove to be malignant [2], head and neck clinician are used to pay attention to differential diagnosis. Despite its rarity, myoepithelioma should be considered in front of a nodule in salivary areas to implement appropriate care and monitoring if necessary, since its malignant counterpart, frequently arising from long-standing tumors, can result in extensive regional destruction and distant metastasis.
The aim of this article is to gather up-to-date datas about epidemiological, clinical and histological features of myoeptihelioma, illustrated by a case report with emphasis on histology and diagnostic approach.
Observations
A 41-year-old female without any relevant medical history apart from a familial history (brother) of leukemia, non-alcoholic and non-smoker was referred to our consultation for a slightly painful mass located on the hard palate, slowly evolving for 1 year. On extra-oral examination, no lymphadenopathy or abnormalities were observed. On intra-oral examination, a one-centimeter, well-circumscribed, firm sessile nodule on the left hemi-palate was observed. No color change or superficial alterations of mucosa were noticed and the tumor did not bleach or pulse on palpation (Fig. 1).
Cone beam computed tomography (CBCT) revealed moderate associated bone involvement, suggesting pressure-related remodeling (Fig. 2) and a non-mineralized composition. No dental infection foci were noticed. Routine hematological tests were prescribed and were within normal range.
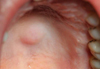 |
Fig. 1 Pre-operative view: well-circumscribed swelling on the left hemi palate, with a sessile base. No change of the overlying mucosa. |
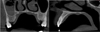 |
Fig. 2 CBCT views showing local cup-shaped bone involvement on coronal and parasagittal views. The tumor is well-delimited within the soft tissues. Of notice: the hyperplastic mucosa of the right sinus with local “air-fluid” levels. |
Surgical management
A complete excision of the lesion was carried out under local anesthesia. After superficial incision, the tumor was easily dissected from the surrounding tissue and presented as a firm, 1 cm well-circumscribed, homogeneously beige lesion (Fig. 3). After complete removal, an additional 1 mm safety margin was removed by curettage of the mucosa and periosteum. Surgical site was closed by interrupted sutures and hemostasis obtained by compression. The specimen was fixed in 10% buffered formaldehyde and submitted to histological examination.
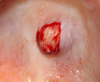 |
Fig. 3 Surgical excision of the tumor disclosing a solid, well-delimited, non-adherent homogeneous mass. The excision was complete. |
Histological examination
Histological examination of the surgical specimen revealed a fully encapsulated tumor composed of plasmacytoid epithelial clusters and very rare salivary ductal components within a mixoid stroma. No cellular atypia, coagulation necrosis or inflammation were noticed. The tumor did not display perineural or periosteal extension as well. Ki-67 index was under 1%. Immunohistostaining was positive for S-100 protein, cytokeratin AE1 and AE3 (Fig. 4) and we found a focal expression of smooth muscle actin (SMA). CD138 and CD163 were negative. Excision of the lesion was complete. Findings were compatible with a benign myoepithelioma.
After one week, wound healing was complete. A regular follow-up, every three months the first year, then twice a year was established to allow an early detection of potential recurrences. After one year follow-up, no recurrence was detected (Fig. 5).
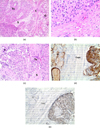 |
Fig. 4 A: Histological view displays a thinly encapsulated tumor (H&E staining, 100×). B: Histological view. Microscopically, homogeneous plasmacytoïd epithelial clusters (H&E staining 400×). C: Histological view showing a rare salivary duct (black arrow) within the tumoral mass. D: Immunostaining positive for cytokeratin AE1–AE3 (healthy gland on the left, ×200). E: Immunostaining positive for PS-100 (healthy gland on the left, ×400). Tu: tumor; S: Stroma; G: healthy Gland; C or Caps: Capsule. |
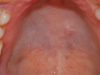 |
Fig. 5 Follow-up at 1 year. No recurrence. Fibrous scar tissue on the surgery site. |
Commentary
Myoepithelioma: clinical features and incidence rate
Accounting for about 1.5% of all salivary gland tumors and 5.7% of all benign minor salivary gland tumors. Incidence is the same in males and females, ranging from 9-85 years old, mostly during the third decade [3]. No risk factors have been clearly identified.
Although myoepithelioma might occur in any tissue with a secretory function (major and minor salivary glands, sweat glands, lacrimal glands, prostate, breast, nasopharynx, lungs, retroperitoneal region, skin and soft tissue) [4] they preferentially develop in the parotid gland (40%) and in minor salivary glands (21%), especially in the hard and soft palate (40% of reported cases) [3]. Those found in minor glands occur in slightly younger individuals, in compare with other sites [2]. Myoepitheliomas display non-specific, variable, clinical features, like other more frequent lesions such as pleomorphic adenoma, which can easily lead to a misdiagnosis. Biopsy is frequently non-specific and fine-needle aspiration cytology possibly misleading. Indeed, depending on the sampling region, myoepithelioma can exhibit the same characteristics as other salivary glands tumors. Architectural examination combined with immunochemical profiles is thus crucial for thorough diagnosis.
Clinically, benign myoepithelioma is usually described as an asymptomatic, insidious mass that slowly enlarges over a period ranging from several months to years, rarely ulcerated (mostly resulting from a chronic local injury). The tumor is well-circumscribed, firm but not hard, and its surface is lobulated without any associated nervous alterations or lymphadenopathy. Originally described as 1–5 cm swellings [5], tumors rarely exceed 3 cm in diameter at the time of the diagnosis. The surgical specimens of myoepitheliomas have a solid, yellow or gray appearance, with glistening cut surface. Apart from the mild pain, the observation in this cases conformed with this description.
Paraclinic examination
Data available about CT and MRI imaging of myoepithelioma are limited. A few recent retrospective studies looked after imaging characteristics of benign and malignant parotid myoepithelioma.
Early findings about benign myoepitheliomas displayed isolated, unilocular (71%) or multilocular (22.2%), mostly round, with smooth contours and weel-defined margins tumors. Capsule are generally discernable on T2-Weighted and contrast enhanced T1-Weighted imaging. Tumors display homogeneous MRI signal intensities, with a T2-Weighted hypersignal [6]. Enhanced CT imaging may display enhancing nodules and nonenhencings areas of linear bands or with cystic configuration [7].
Some case report describe central benign myoepithelioma located in the maxilla [8] or pressure-related bone resorption of the mandible associated to adjacent sublingual benign myoepithelioma resulting in a round, lobulated, well-circonscribed radiolucency discernable on orthopantomogram or CBCT [9].
Histological features
Myoepithelioma exhibits the variable nature of myoepithelial cells through the great variability of its structure and components from an area to another and from a tumor to another. Accordingly, biopsy is frequently non-specific and fine-needle aspiration cytology possibly misleading [10]. Indeed, depending on the sampling region, myoepithelioma can exhibit the same characteristics as other salivary glands tumors. A complete resection of the tumor is necessary for appropriate histological examination.
Previous descriptions suggest that myoepitheliomas can either develop with a full capsule (for the parotid myoepithelioma), or with an irregular capsule [11] (with few interruption zones) or without a capsule (mostly described for palatal myoepithelioma) [12]. The tumor observed in this case was fully encapsulated in a fibrous capsule, in accordance with other description in the same area [4,13] (Fig. 3).
Cells types described in myoepithelioma include spindle, plasmacytoid (or hyaline), epithelioid, clear-cell and oncocytic types. Spindle and plasmacytoid are the most reported variants. Plasmacytoid type is frequent in minor salivary glands, whereas spindle cells are often reported in the parotid [4,5] Another recently described entity, called “mucinous myoepithelioma” displays intracellular mucin material [14]. Clear cells type can undergo cystic changes [15]. One type or mixture of different cell-type can be found in the same tumor. Plasmacytoid myoepithelioma should also be distinguished from rhabdomyoma and oncocytoma, the latter displaying granular cytoplasm on histological examination. Growth pattern may be solid, myxoid or reticular and sometimes found together within the same neoplasm. In this case, the stroma was myxoid (“pleomorphic-adenoma-like”), a seemingly rare variant in compare with non-myxoid myoepitheliomas, which made the immunostaining profile essential for diagnosis.
According to the widest immunohistochemical study (101 cases), SMA appears to be inconstantly expressed (36%), despite the tumor denomination “myoepithelioma”. Pancytokeratin AE1/AE3 (93%) and myoepithelial markers like calponin (86%), PS-100 (87%) are generally significantly positive [16]. Cytokeratins CK7 and CK14 and myoepithelial marker p63 are usually positive [4,1] Markers expressions vary depending on the tumoral subtype. For example, the epitheloid subtype could express more epithelial marker than other subtypes, that mostly express myoepithelial markers [17].
In this case, S100, AE1-AE3 and SMA immunostaining were performed and positive. We found a focal expression of SMA. Put together with histomorphological data, the immunostaining profile displayed allowed a reliable diagnosis of myoepithelioma.
Surgical management
Recommended surgical management of myoepithelioma is a complete surgical excision with a safety margin of uninvolved tissue [4]. Clinician should pay attention the peripheral detachment of the tumor during the surgery to detect potential extension or invasive behavior of the tumor. During the surgical management of this patient, the tumor was easily separated from the surrounding tissue after superficial incision, since no adherence were noticeable.
To provide guidance for the surgery management, extemporaneous examination of the specimen − which could not be performed under the chosen operating conditions for this case − is a preferable option, when possible, in compare with preoperative fine needle aspiration or biopsy which could underestimate the tumor nature and behavior.
Prognosis and recurrence
The prognosis of benign myoepithelioma is often reported similar to mixed tumors [18], though Gore et al. reported a recurrence rate from 15–18% [19]. No distant dissemination has been reported yet for benign myoepithelioma. Up to now, no particular cell-type or growth pattern have proved to have a significant prognosis impact. Incomplete resection of the tumor seems to result in a higher recurrence rate [20].
Main differential diagnoses: malignant myoepithelioma and other salivary gland tumors
Malignant myoeptihelioma
Given the histological aspect, the malignant counterpart of myoepithelioma, also called “myoepithelial carcinoma” (MC) was the main differential diagnosis. MC accounts for 0.2–0.6% of all salivary gland tumors, affecting equally men and women of a wide range of ages, the mean age being slightly higher than the benign ones (55-years-old) [2]. Up to now, risk factors have not been clearly identified. The parotid gland is the most common primary site (75%), followed by the submandibular gland and minor salivary glands [2]. More unusual locations have also been reported including, gingiva, larynx, lateral wall of the nasopharynx, base of the tongue, maxillary sinus and cavernous sinus or more distant extra-oral sites [21–24].
It typically present as an unencapsulated soft to firm masse, ranging from 2–10 cm . The cut surface is grey to tan white and occasionally hemorrhagic, with cystic degeneration and necrosis [3].
They may arise de novo (30–40%) but at least 50% develop from a preexisting pleomorphic adenoma or myoepithelioma, especially in long standing tumors or in tumors with multiple recurrences [25,3]. MCs arising from the proliferation of the myoepithelial component of a epithelial-myoepithelial carcinoma has also been described [26].
Diagnosis of MC is based on two major histologic criteria: unequivocally malignant and exclusively myoepithelial [6,23] This suggest a lack of ductal or acinar differentiation and allow a differential diagnosis with epithelial-myoepithelial carcinoma. Typical features of malignant myoepithelioma include multinodular architecture, infiltrative and destructive growth − particularly inside the surrounding glandular tissue − with unclear delimitation or nervous unsheathing, cellular pleomorphism, necrotic areas, hypercellular peripheral rims [27]. and high mitotic index (Ki-67 > 10%) support a malignant nature of the tumor. Latest data suggest a high recurrence rate and metastatic potential for these tumors [28] with a propensy for distant (mostly lung) metastasis rather than regional lymph node metastasis [3]. Prognosis seems to be negatively correlated with tumor necrosis [29]. Tumors tend to be more aggressive with short clinical course when they arise de novo [30]. Presence of preexisting carcinoma ex-pleomorphic adenoma and vascular invasion could negatively impact disease-free-survival [28]. They are considered intermediate to high grade carcinomas. Accordingly, radiotherapy and chemotherapy after surgical management and precise identification of tumor extension with CT, MRI and/or PET-CT are frequently implemented, though very few data are available yet for this tumor [31]. Latest WHO report suggests that one third of the patients are cured with surgical management alone, and one third experience metastatic and progressive disease [3], suggesting that the third left have multiple recurrences [3].
Regarding CT imaging characteristics, Yue et al. gathered retrospectively datas from 10 patients with MC from various origins (1 palatine myoepithelioma). In compare with benign myoepithelioma, solitary lesions had all irregular shapes, lobulated or multinodular, with a partially ill-defined boundary consistent with infiltrative tumor border and incomplete capsule. Nonenhanced CT showed inhomogeneous density corresponding to mucoid material or calcifications in 2 cases. They also reported inhomogeneous enhancement with small cystic (necrotic or mucus regions), slit-like (interstitial hemorrhage) and nodular (abundant vascular epithelial network) enhancements [32].
Pleomorphic adenoma and other salivary gland tumors
In this observation, the growth rate of the tumor was reassuring regarding high-grade malignant tumors. Given the clinical aspect, pleomorphic adenoma (PA) was the main differential diagnosis.
PA is the most common salivary gland tumor of the palate accounting for approximately half of all salivary neoplasms and 40–80% of all minor salivary gland tumors, with a major predilection for the parotid glands [33]. In order to histologically distinguish PA from the rare diagnosis of myoepithelioma, authors all agree to say that no chondroid, osteoid or fibromyxoid stroma should be observed in myoeptheliomas. Fibromyxoid stroma would indicate PA. Besides, epithelial component of myoepitheliomas should account for less than 5–10% [19]. Most authors consider that myoepithelioma can display some rare ductal compounds (1 per field at ×200–×400 magnification or 5% of tumor parenchyma) which can be used to distinguish them from mixed tumors [34]. In this case, stroma was exclusively myxoid and few isolated ducts (<1 per field at 200 magnification) were found.
In 1998, WHO suggested that myoepitheliomas have a more aggressive behavior in compare with pleomorphic adenomas. Subsequent publications, including 2017 WHO classification of Head and Neck Tumors, are not so categorical regarding the difference of behavior between these tumors.
Polymorphous low-grade adenocarcinoma, basal cell adenocarcinoma, epithelial-myoepithelial carcinomas, low-grade mucoepidermoid carcinoma, adenoid cystic carcinoma and the recently described mammary analog secretory carcinoma of the salivary gland (MASCSG) were also considered and excluded based on structural and/or cytologic criteria or immunostaining profiles.
Further differential diagnosis
CBCT imaging allowed to exclude infection related diseases, bone tumors, odontogenic tumors and palatine torus. No further imaging investigation appeared to be necessary.
Connective tissue tumors, especially myxoma, leiomyoma, schwannoma and neurofibroma (and less likely sialolipoma) were also possible. Leiomyoma, myxoma are PS-100 negative. Scwhannoma, neurofibroma and sialolipoma display highly specific histological features and were easily excluded on microscopic characterization.
Plasmacytoid cell observed in this case were similar to neoplastic plasma cells of plasmacytoma. For this reason we ran CD138 immunostaining, which was negative. CD163 was also negative, excluding histiocyte-mediated diseases.
Oncocytoma, oncocytic hyperplasia, or metastatic lesion were also included in the differential diagnosis.
Figure 6 summarize the chosen approach to treat this patient and gather the main clinical and histological features.
 |
Fig. 6 Diagnostic approach implemented in this case. |
Conclusion
Palate is at the crossroad of many different types of tumors. Among them, minor salivary glands tumors show a considerable variability and share many similarities at the same time, making them clinically and histologically uneasy to diagnose and characterize. Despite its rarity myoeptihelioma should be hypothesized in front of a palatine sessile nodule. Discovery of such tumor is often incidental and diagnosis is histological. Accordingly, clinician and pathologist should be particularly cautious about differential diagnosis and benign/malignant status of the tumor and be careful to follow-up, since few data are up-to-now available.
Conflicts of interests
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
References
- Seifert G, Sobin LH. The World Health Organization’s histological classification of salivary gland tumors. A commentary on the second edition. Cancer 1992;70:379–385. [CrossRef] [PubMed] [Google Scholar]
- Eveson JW, Auclair P, Gnepp DR, El-Naggar AK. Tumours of the salivary glands. Introduction. In: Barnes L, Eveson JW, Reichart P, Sidransky D, Eds. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours, IARC Press Lyon, 2005. [Google Scholar]
- EI-Naggar AK, Chan JKCG, Randis JR, Takata T, Slootweg PJ, Eds. WHO Classification of head and neck tumours, 4th edition, Lyon: IACR, 2017. [Google Scholar]
- Nair BJ, Vivek V, Sivakumar TT, Joseph AP, Varun BR, Mony V. Clear cell myoepithelioma of palate with emphasis on clinical and histological differential diagnosis. Clin Pract 2014;4:628. [PubMed] [Google Scholar]
- Simpson RH, Jones H, Beasley P. Benign myoepithelioma of the salivary glands: a true entity? Histopathology 1995;27:1–9. [CrossRef] [PubMed] [Google Scholar]
- Ding J, Wang W, Chen T et al. MRI and CT imaging characteristics of myoepithelioma of the parotid gland. Acta Radiol 2016;57:837–843. [CrossRef] [PubMed] [Google Scholar]
- Wang S, Shi H, Wang L, Yu Q. Myoepithelioma of the parotid gland: CT imaging findings. AJNR Am J Neuroradiol 2008;29:1372–1375. [Google Scholar]
- Gil MC, Bucci T, Cuella CN et al. Intraosseous myoepithelioma of the maxilla: clinicopathologic features and therapeutic considerations. J Oral Maxillofac Surg 2008;66:800. [CrossRef] [PubMed] [Google Scholar]
- Hunt KT, Stevens MR, Nguyen CT et al. Benign myoepithelioma of floor of mouth with mandibular involvement: a case report and literature review. J Oral Maillofac Surg 2011;69:3001–3005. [CrossRef] [Google Scholar]
- Irving J, Mejıa-Hernandez M, Valdez AC, Leo DD et al. Malignant myoepithelioma of the soft palate. Auris Nasus Larynx 2013;40:231–234. [CrossRef] [PubMed] [Google Scholar]
- Barnes L, Appel BN, Perez H, El-Attar AM. Myoepitheliomas of head and neck: case report and review. J Surg Oncol 1985;28:21–28. [CrossRef] [PubMed] [Google Scholar]
- Turgut S, Cekic A, Ergul G et al. Myoepithelioma of the parotid gland: a report of two cases. Ear Nose Throat J 2001;80:155–158. [PubMed] [Google Scholar]
- Bahi S, Warter A, Feki A. Myoepithelioma of minor salivary glands: a case report. Med Buccale Chir Buccale 2003;9:113–118. [CrossRef] [Google Scholar]
- Gnepp DR. Mucinous myoepithelioma: a recently described new myoepithelioma variant. Head Neck Pathol 2013;7:S85–S89. [CrossRef] [PubMed] [Google Scholar]
- Agarwal AK, Sethi A, Chopra S, Sareen D. Clear cell myoepithelioma of the hard palate. Internet J Head Neck Surg 2007;2(2). [Google Scholar]
- Hornick JL, Fletcher CD. Myoepithelial tumours of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol 2003;27:1183–1196. https://doi.org/10.1007/s10006-012-0324-y. [CrossRef] [PubMed] [Google Scholar]
- Kasamatsu A, Shiiba M, Nakashima D et al. Epitheloid myoepithelioma of the hard palate. Oral Maxillofac Surg 2013;17:63–66. [CrossRef] [Google Scholar]
- Just PA, Miranda L, Elouaret Y, Meatchi T, Hans S, Badoual C. Classification of salivary gland tumors. Ann Otolaryngol Head Neck Surg 2008;125:331–340. [Google Scholar]
- Gore CR, Panicker NK, Chandanwale SS, Singh BK. Myoepithelioma of minor salivary glands − a diagnostic challenge: report of three cases with varied histomorphology. J Oral Maxillofac Pathol 2013;17:257–260. [CrossRef] [PubMed] [Google Scholar]
- Maiorano E, Altini M, Flavia G. Clear cell tumors of salivary glands, jaws and oral mucosa. Semin Diagn Pathol 1997;14:203–212. [PubMed] [Google Scholar]
- Kutzner H, Mentzel T, Kaddu S, Soares LM, Sangueza OP, Requena L. Cutaneous neoplasm composed of myoepithelial cells. Am J Surg Pathol 2001;25:348–355. [CrossRef] [PubMed] [Google Scholar]
- Martinez-Madrigal F, Santiago PH, Meneses A, Dominguez MH, Royas ME. Plasmocytoid myoepithelioma of the laryngeal region: a case report. Hum Pathol 1995;26:802–804. [CrossRef] [PubMed] [Google Scholar]
- Hong Y, Song XG, Chen S et al. Rapid-developed primary malignant myoepithelioma in the cavernous sinus: a case report. BMC Neurol 2013;13:1–6. [CrossRef] [PubMed] [Google Scholar]
- Bigotti G, Di Giorgio GG. Myoepithelioma of the breast: histologic, immunologic and electromicroscopic appearance. Ultrastruc Pathol 1995;19:269–274. [CrossRef] [Google Scholar]
- Suba Z, Né meth Z, Gyulai-Gaál S, Ujpal M, Szende B, Szabó G. Malignant myoepithelioma. Clinicopathological and immunohistochemical characteristics. Int J Oral Maxillofac Surg 2003;32:339–341. [CrossRef] [PubMed] [Google Scholar]
- Cornolti G, Ungari M, Morass ML et al. Amplification and over expression of HER2/neu gene and HER2/neu protein in salivary duct carcinoma of the parotid gland. Arch Otolaryngol Head Neck Surg 2007;133:1031–1036. [CrossRef] [PubMed] [Google Scholar]
- Santos KCPP, Matsuzaki H, Unetsubo T, Tsuyoshi S, Nagatsuka H, Asaumi JI. De novo myoepithelial carcinoma with multiple metastases arising from a submandibular salivary gland: a case report. Oncol Lett 2017;13:2679–2683. [CrossRef] [PubMed] [Google Scholar]
- Kong M, Drill EN, Morris L, West L, Klimstra D, Gonen M, Ghossein R, Katabi N. Prognostic factors in myoepithelial carcinoma of salivary glands: a clinicopathologic study of 48 cases. Am J Surg Pathol 2015;39:931–938. [CrossRef] [PubMed] [Google Scholar]
- Passador-Santos F, Grönroos M, Irish J et al. Clinicopathological characteristics and cell cycle proteins as potential prognostic factors in myoepithelial carcinoma of salivary glands. Virchows Arch 2016;468:305–312. [CrossRef] [PubMed] [Google Scholar]
- Mejıa-Hernandez J et al. Malignant myoepithelioma of the soft palate. Auris Nasus Larynx 2013;40:231–234. [CrossRef] [PubMed] [Google Scholar]
- Ettl T, Schwarz-Furlan S, Gosau M, Reichert TE. Salivary gland carcinomas. Oral Maxillofac Surg 2012;16:267–283. doi:10.1007/s10006-012-0350-9. [CrossRef] [Google Scholar]
- Yue D, Feng W, Yahong L et al. Myoepithelial carcinoma of the salivary gland: pathologic and CT imaging characteristics: reports of 10 cases and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol 2017;123:e182–e187. [CrossRef] [PubMed] [Google Scholar]
- Clauser L, Mandrioli S, Dallera V, Sarti E, Galiè M, Cavazzini L. Pleomorphic adenoma of the palate. J Craniofac Surg 2004;15:1026–1029. [CrossRef] [PubMed] [Google Scholar]
- Fornias Sperandio F, Salgueiredo Giudice F, Cantanhede Orsini Machado de Sousa S. Myoepithelioma of the soft palate: a case report giving special attention to the differential diagnosis. J Oral Maxillofac Res 2011;2:e4. [Google Scholar]
All Figures
 |
Fig. 1 Pre-operative view: well-circumscribed swelling on the left hemi palate, with a sessile base. No change of the overlying mucosa. |
| In the text | |
 |
Fig. 2 CBCT views showing local cup-shaped bone involvement on coronal and parasagittal views. The tumor is well-delimited within the soft tissues. Of notice: the hyperplastic mucosa of the right sinus with local “air-fluid” levels. |
| In the text | |
 |
Fig. 3 Surgical excision of the tumor disclosing a solid, well-delimited, non-adherent homogeneous mass. The excision was complete. |
| In the text | |
 |
Fig. 4 A: Histological view displays a thinly encapsulated tumor (H&E staining, 100×). B: Histological view. Microscopically, homogeneous plasmacytoïd epithelial clusters (H&E staining 400×). C: Histological view showing a rare salivary duct (black arrow) within the tumoral mass. D: Immunostaining positive for cytokeratin AE1–AE3 (healthy gland on the left, ×200). E: Immunostaining positive for PS-100 (healthy gland on the left, ×400). Tu: tumor; S: Stroma; G: healthy Gland; C or Caps: Capsule. |
| In the text | |
 |
Fig. 5 Follow-up at 1 year. No recurrence. Fibrous scar tissue on the surgery site. |
| In the text | |
 |
Fig. 6 Diagnostic approach implemented in this case. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


