| Issue |
J Oral Med Oral Sug
Volume 24, Number 3, 2018
|
|
|---|---|---|
| Page(s) | 133 - 137 | |
| Section | Cas clinique et revue de la littérature / Up-to date review and case report | |
| DOI | https://doi.org/10.1051/mbcb/2017042 | |
| Published online | 10 October 2018 | |
Up-to Date Review And Case Report
Adenosquamous carcinoma, a rare and unknown tumor
1
Paul Sabatier University, Dental Faculty, CHU Toulouse, France
2
INSERM U1043, Université Toulouse III CHU Purpan,
BP 3028,
31024
Toulouse, France
3
Department of Oral surgery, Dental Faculty, Université Paul Sabatier, Centre hospitalo-universitaire de Toulouse,
31062
Toulouse, France
4
Otorhinolaryngology Department,
24 Chemin de Pouvourville,
31400
Toulouse, France
5
Pathology Department,
1 Av. Irene Joliot-Curie,
31100
Toulouse, France
6
Université de Toulouse, UPS, INP, LAPLACE,
118 route de Narbonne,
31062
Toulouse, France
* Correspondence: benat.g@chu-toulouse.fr
Received:
1
June
2017
Accepted:
26
December
2017
Introduction: Adenosquamous carcinoma (ASC) of the head and neck is a rare malignant tumor, with one hundred cases diagnosed so far in the literature. Observation: A 66 years old female patient had a ASC of the right posterior floor of the mouth with a classification T1N0M0. Treatment consisted of surgical management and active surveillance. Discussion: Through this case report, we present this type of tumor and we discuss the main differential diagnosis from a clinical and histological point of view. Conclusion: This entity was described for the first time in 1968 by Gerughty. The CAS is defined by the World Health Organization as “a tumor of the upper respiratory tract, with two distinct squamous and glandular components.” The CAS is considered as an aggressive tumor with a redoubtable prognosis should not be ignored.
Key words: oral surgery / adenosquamous carcinoma / pathology / oncology / buccal mucosa / rare tumor
© The authors, 2018
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Adenosquamous carcinoma (ASC) is a rare and malignant tumor of the head and neck region. Approximately 100 cases have been reported in the literature. This entity was first described in 1968 by Gerughty [1]. ASC is defined by the World Health Organization as “a malignant tumor of the upper aerodigestive tract, which has two distinct squamous and glandular contingents.” The squamous part is usually predominant. It can manifest as a well differentiated or a poorly differentiated tumor, and may occur as a carcinoma in situ or an invasive tumor. The glandular component is most often found in the deeper layer of the tumor. ASC is an aggressive tumor with a poor prognosis with frequent local and locoregional metastases despite appropriate treatment [2].
Clinical case
A 66-year-old woman with no relevant medical or surgical history consulted us with complaints of persistent pain under the tongue. She was an active smoker at 40 pack years without any history of alcoholism. The pain had gradually worsened over the course of 4 years. Intraoral examination revealed a wide erythematous region of the right posterior floor, rising to the lingual slope of the distally edentulous mandibular crest of 44. There was an ulceration in the area between the floor of the mouth (FOM) and the ventral surface of the tongue. The ulceration was localized on the erythematous area, surrounded by keratotic areas that were whitish, inhomogeneous, and somewhat thick (Fig. 1). Although the lesion was tender, it did not bleed on contact and was not indurated. The lingual function examination was normal, and no swallowing or phonation disturbances were noted. The extraoral exam was normal and no lymphadenopathy or swellings were noted.
Due to the inhomogeneous aspect of the lesion, a biopsy was performed. The histopathological analysis of the biopsy made the diagnosis of ASC (Fig. 2). The entire biopsy sample consisted of tumor material and showed the presence of a well-differentiated epidermoid component; one part was a carcinoma in situ and the other part was invasive, with a keratinized area and some keratin pearls. The glandular contingent consisted of four glands. There was also a large inflammatory infiltrate and the existence of many mitotic figures Staining with periodic acid/Schiff (PAS) and alcian blue confirmed the secretory nature of these glands.
The patient was therefore referred to the head and neck cancer surgery department. In the preoperative assessment at the department, she underwent a pan-endoscopy of the upper aerodigestive tract, a cervicofacial and thoracic CT scans, and a cervicofacial MRI to accurately characterize the lesion (8 mm transverse diameter by 10 mm vertical diameter). The tumor was adjacent to the mandible without bone infiltration and there was no sign of lymph node or distant metastases. The lesion was classified as a cT1N0M0. Surgical intervention was decided upon at a multidisciplinary consultation meeting. A right posterior uninterrupted pelvi-mandibulectomy of
3.5 × 5.5 × 1.5 cm was performed associated with a complete ipsilateral cervical dissection, followed by posterior-based buccinator myomucosal flap reconstruction.
The histopathological analysis of the specimen confirmed the adenosquamous nature of the lesion with an ulcerated superficial, infiltrating tumor with a double component of massive, trabecular and glandular, cribriform architecture. It presented with a contingent of moderately differentiated squamous cell carcinoma with slight keratinization andmasses of eosinophilic cells, which were surrounded, at some instances, by keratinization foci. Alcian blue staining revealed a glandular mucosecretory component in the deeper layers of the infiltrating tumor.The tumor had a 14 mm diameter at its major axis and showed capsular invasion of 1 mm. The stromal reaction was fibroinflammatory. There were many atypical mitotic figures (Fig. 3). Immunohistochemical analysis showed CK5/6 and CK7 positivity for the glandular component, and CK5/6 positivity and CK7 negativity for the epithelial component (Figs. 4 and 5). A test for human papillomavirus (HPV) was negative. The histopathological analysis classified the tumor as a pT1N0M0 R0 lesion (52 lymph nodes 103 analyzed) with a posterior section in dysplastic zone.
A complementary surgical procedure was therefore performed to widen the anterior and posterior margins. The analysis of the specimen did not find any tumor residuals, ruling out indications for adjuvant chemoradiotherapy treatment. The strict postoperative monitoring consisted of one clinical follow-up every 2 months over a 1-year period. Subsequently, the consultation frequency will be progressively spaced out. Regular computed tomography scans will be required as a means of radiological follow-up. This will occur every 6 months for 3 years because of the high risk of early relapse.
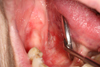 |
Fig. 1 Macroscopic view of the lesion. |
 |
Fig. 2 Biopsy − LEFT (hematoxylin & eosin staining − HE) keratin pearls (blue arrows) and RIGHT (alcian blue − AB) with AB-positive region confirming the secretory nature of the glands (green arrows). |
 |
Fig. 3 Surgical piece – LEFT: HE: ulcerated surface (blue triangle) of the tumor and RIGHT: AB: AB-positive in the deeper invasive tumor gland (green arrow). |
 |
Fig. 4 Surgical specimen – LEFT: CK5/6 positivity for the glandular component and for the epithelial component and RIGHT: CK7 positivity only for the glandular component. |
 |
Fig. 5 Surgical specimen – LEFT: CK5/6 and RIGHT CK7 – showing the presence of two distinct cell types. |
Discussion
ASC is considered an aggressive tumor with a guarded prognosis with up to 80% of patients having metastases [1]. Our patient had been reporting painful tumor episodes at more or less regular intervals for 4 years. Sheahan associates this symptomatology with frequent perineural invasions (found in 75% of the cases) [3], although they were not found in this case. The tumor was classified as cT1N0M0 under TNM staging, which was confirmed by histopathological analysis (pT1N0M0). The sex ratio (male: female) is usually between 9:1 [1] and 2:1 [4]. The age of occurrence in the present case corresponds to the median age of 60 years reported previously. [2].
The oral cavity is the second most common location after the larynx [2]. The histopathological characteristics of this tumor and now well described. Unlike some previous report where the diagnosis was made only with the aid of a substantial immunohistochemical panel, [5,6] few antibodies were used for diagnosis in this case. The selective positivities CK5/6 and CK7 and the characteristic morphological features (superficial epithelial component, deep glandular) allowed for an early diagnosis. Thorough pathological examination, along with the known and compatible histopathological features, the histopathologist will seek to confirm the diagnosis by immunohistochemical examination. However, there is still doubt regarding whether the tumor had an epithelial or a salivary origin. Since its first description in 1968, when Gerughty [1] attributed a salivary origin to it, ASC was linked to the mucoepidermoid carcinoma (MEC). It was in 1984 that Evans [7] suggested separating these two entities in view of the poor prognosis of ASC, unlike that of MEC. The origin of this tumor is still being debated, and the evidence is pointing towards an epithelial origin. In 1991 Ellis [8] suggested an unprecedented double origin of ASC, i.e., both salivary and epithelial. However, Napier [9] and Fonseca [10] have reported that origin is strictly epithelial.
Nevertheless, despite the precise histopathological description, this tumor may be incorrectly diagnosed. In effect, a superficial biopsy will not be able to reveal the glandular component. Moreover, its low frequency makes it possible to be confused with a MEC (Seethala states that 20% of high-grade MECs are in fact ASCs [11]), adenoid squamous cell carcinoma (ASCC) or basaloid squamous cell carcinoma (BSCC). In ASC, the squamous component is morphologically identical to MEC with the presence of keratinization foci. The glandular component is always formed from duct-like structures with or without mucus cells. These two components are always well separated. MEC is a salivary tumour with no macroscopic mucosal involvement. In the histopathological analysis of a high-grade MEC, a predominant presence of intermediate cells is observed without keratinization of any clear cells. In addition, glandular and squamous components show an overlap (Tab. I).
ASCC features both a component-type epidermoid carcinoma and a squamous cell carcinoma on the tumor surface, along with a pseudoglandular part, which corresponds to cells or groups of cells in acantholysis, with cellular debris predominating in these pseudoglandular lumens. The presence of clear cells and inflammatory infiltrate is common (Tab. II). The BSCC is similar in appearance to ASC with PAS or alcian blue-positive squamous or glandular differentiation zones. Nevertheless, BSCC has basaloid solid cell nests with a central comedonecrosis region, which is never present in ASCC.
Histopathological comparison of ASC and MEC.
Histopathological comparison of ASC and ASCC.
Conclusion
ASC is a rare tumor of the upper aerodigestive tract that usually progresses rapidly and aggressively. The major differential diagnosis is MEC, ACC, and BSCC.
Conflict of interest
The authors declare that they have no conflicts of interest in relation to this article
References
- Gerughty RM, Hennigar GR, Brown FM. Adenosquamous carcinoma of the nasal, oral and laryngeal cavities. A clinicopathologic survey of ten cases. Cancer 1968;22:1140–1155. [CrossRef] [PubMed] [Google Scholar]
- Schick U, Pusztaszeri M, Betz M, et al. Adenosquamous carcinoma of the head and neck: report of 20 cases and review of the literature. Oral Surg Oral Med Oral Pathsol Oral Radiol 2013;116:313–320. [CrossRef] [Google Scholar]
- Sheahan P, Fitzgibbon J, Lee G, et al. Adenosquamous carcinoma of the tongue in a 22-year-old female: report of a case with immunohistochemistry. Eur Arch Otorhinolaryngol 2003;260:509–512. [CrossRef] [PubMed] [Google Scholar]
- Keelawat S, Liu CZ, Roehm PC, et al. Adenosquamous carcinoma of the upper aerodigestive tract: a clinicopathologic study of 12 cases and review of the literature. Am J Otolaryngol 2002;23:160–168. [CrossRef] [PubMed] [Google Scholar]
- Lima CF, Acay R, Anbinder AL, Almeida JD, Carvalho YR. Oral adenosquamous carcinoma mimicking a pyogenic granuloma: a challenging diagnosis. Braz Dent J 2016;27:781–786. [CrossRef] [PubMed] [Google Scholar]
- Sravya T, Rao G, Kumar M, Sudheerkanth K. Oral adenosquamous carcinoma: Report of a rare entity with a special insight on its histochemistry. J Oral Maxillofac Pathol 2016;20:548. [CrossRef] [Google Scholar]
- Evans HL. Mucoepidermoid carcinoma of salivary glands: a study of 69 cases with special attention to histologic grading. Am J Clin Pathol 1984;81:696–701. [CrossRef] [PubMed] [Google Scholar]
- Ellis GL, Auclair P, Gnepp DR, et al. Adenosquamous Carcinomas. Surgical Pathology of the Salivary Glands. Philadelphia, PA: WB Saun-ders, 1991:455–459. [Google Scholar]
- Napier SS, Gormley JS, Newlands C, et al. Adenosquamous carcinoma. A rare neoplasm with an aggressive course. Oral Surg Oral Med Oral Pathol Oral Radiol 1995;79:607–611. [CrossRef] [Google Scholar]
- Fonseca FP, Ramos LMA, Vargas PA, et al. Oral adenosquamous carcinoma: evidence that it arises from the surface mucosal epithelium: correspondence. Histopathology 2012;61:321–323. [CrossRef] [PubMed] [Google Scholar]
- Seethala RR, Dacic S, Cieply K, et al. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol 2010;34:1106–1121. [CrossRef] [PubMed] [Google Scholar]
All Tables
All Figures
 |
Fig. 1 Macroscopic view of the lesion. |
| In the text | |
 |
Fig. 2 Biopsy − LEFT (hematoxylin & eosin staining − HE) keratin pearls (blue arrows) and RIGHT (alcian blue − AB) with AB-positive region confirming the secretory nature of the glands (green arrows). |
| In the text | |
 |
Fig. 3 Surgical piece – LEFT: HE: ulcerated surface (blue triangle) of the tumor and RIGHT: AB: AB-positive in the deeper invasive tumor gland (green arrow). |
| In the text | |
 |
Fig. 4 Surgical specimen – LEFT: CK5/6 positivity for the glandular component and for the epithelial component and RIGHT: CK7 positivity only for the glandular component. |
| In the text | |
 |
Fig. 5 Surgical specimen – LEFT: CK5/6 and RIGHT CK7 – showing the presence of two distinct cell types. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


