| Issue |
J Oral Med Oral Surg
Volume 31, Number 3, 2025
|
|
|---|---|---|
| Article Number | 24 | |
| Number of page(s) | 7 | |
| DOI | https://doi.org/10.1051/mbcb/2025029 | |
| Published online | 05 August 2025 | |
Case Report
Cerebro-facial arteriovenous metameric syndrome: report of a rare case with involvement of muscles of mastication
1
Maulana Azad Institute of Dental Sciences, MAMC Complex, New Delhi, India
2
Maulana Azad Medical College and Lok Nayak Hospital, New Delhi, India
* Correspondence: calmshikhs@yahoo.com
Received:
6
January
2025
Accepted:
23
June
2025
Cerebro-facial metameric syndrome (CAMS) is a rare entity comprising of vascular malformations of craniofacial structures in a metameric distribution. It is classified into 3 subtypes depending upon metamere involved into medial prosencephalic, lateral prosencephalic and rhombencephalic group. Retinal involvement is most commonly seen in cases reported with facial vascular malformations. Facial lesions are generally silent or may present as small, angiomatous lesion at the time of presentation. Here, we report a case of CAMS 1 & 2 presenting with facial asymmetry, and intraoral lesions which was diagnosed as CAMS on contrast enhanced computed tomography (CECT), and CT Angiography. CECT Angiography demonstrated involvement of muscles of mastication with presence of phleboliths, feeder vessels and draining veins. Magnetic resonance imaging (MRI) Brain with Angiography was done for intracranial lesions.
Key words: CAMS / cerebro-facial / malformation / arteriovenous / AV malformation
© The authors, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cerebrofacial arteriovenous metameric syndrome (CAMS) is rare and includes vascular malformations of the craniofacial region in a metameric distribution [1–3]. Metameric syndromes have varied phenotypes and primarily involve the arteries or veins. It is due to dysfunction in a metamere resulting from somatic mutation prior to the migration and differentiation of neural crest cells, giving rise to vascular malformations [1,2,4].
It has three subtypes: medial prosencephalic group, lateral prosencephalic group, and rhombencephalic group, depending on the involvement of a metamere and the distribution of the lesion [1]. Clinically, it includes soft tissue and intracranial lesions and can be focal or widespread [5]. Although clinical presentation is sufficient to arrive at a diagnosis in more common lesions such as Sturge-Weber syndrome, imaging is essential for diagnosis to identify arteriovenous malformations along a metamere in rare cases of CAMS. Undiagnosed intracranial lesions may cause significant morbidity; therefore, it is necessary to rule out CAMS in cases of facial arteriovenous malformations.
The present case report describes the imaging findings in a case of cerebrofacial arteriovenous metameric syndrome type 1 and 2, with manifestations of oral lesions, involvement of the muscles of mastication, and phleboliths, without involvement of the retina.
Case report
A 21-year-old female presented to the Department of Oral Medicine and Radiology at a Tertiary Care Dental Hospital with the chief complaint of multiple small red nodules inside her oral cavity for the past 8 years. Her past medical and dental history were non-contributory. There was no previous history of trauma, and the lesions were not associated with any symptoms. Extraoral examination showed facial asymmetry with diffuse facial swelling on the left side, extending from the infraorbital margin to the region of the lower lip, with obliteration of the left nasolabial fold and drooping of the left angle of the mouth (Fig. 1). The overlying skin was normal in colour and texture, and afebrile to touch. Palpatory findings revealed a dome-shaped, soft, non-tender swelling in the left postauricular region, approximately 1 × 1 cm in size.
On intraoral examination, two round to oval, bluish, non-compressible, non-reducible nodular swellings were present, the largest measuring approximately 1.5 × 1.5 cm, on the left buccal mucosa and adjacent labial mucosa, with a characteristic bruit (Fig. 2A). Diffuse bluish discoloration was present in the left pterygomandibular raphe region (Fig. 2B). Further examination of the oral cavity revealed serpiginous reddish-purple lines in the mid-palatal region, slightly toward the left side (Fig. 2C). Haematological findings were within normal limits. An orthopantomogram showed multiple focal calcifications with characteristic concentric laminations, giving a target-eye appearance at the outer border of the left mandibular ramus and condylar process, suggestive of phleboliths (Fig. 3). Based on the clinical and radiographic features, a provisional diagnosis of arteriovenous malformation involving multiple sites in the oral cavity was made.
Computed tomography (CT) Angiography was performed after intravenous contrast injection on 128 slice MDCT scanner followed by Multiplanar reformation. CT showed multiple dilated enhancing serpiginous vascular channels within extending along the left temporal region, infratemporal fossa, masticator space and left parapharyngeal space. Left side muscle of mastication including masseter, medial and lateral pterygoid, temporalis and left buccinator appeared bulky (Figs. 4A-4C). Multiple well defined round nodular calcific foci with central lucency are seen anterior to the left carotid sheath, left parapharyngeal space, masticator space, left lateral pterygoid and masseter muscle largest measuring 7 × 7.5 mm in left parapharyngeal space suggestive of phleboliths (Figs. 5A-5C). Arterial phase opacification with associated prominence was seen of draining facial veins including the left anterior facial, superficial temporal, anterior and posterior branches of the posterior facial veins, anterior jugular and external jugular veins suggestive of early draining veins (Figs. 6A-6C). Arterial supply is seen from the left superficial temporal, internal maxillary and posterior auricular branches of the left external carotid artery (Figs. 6D-6F). Multiple dilated vessels were seen along the left medial thalamus in the region of the internal cerebral vein with early opacification of straight sinus, left transverse sinus, sigmoid sinus, and internal jugular vein (Figs. 7A-7C). MRI Brain with Angiography was performed on 3T magnet system. Magnetic Resonance Imaging (MRI) of the brain show dilated serpiginous vascular channels in the region of the internal cerebral vein and vein of Galen showing T2 flow voids within and FLAIR hyperintensity along the muscles of mastication on the left side (yellow arrows) with well-defined nodular hypointense foci within (Figs. 8A-8C). MR angiography of the brain reveals communication between a branch from the P2 segment of the left posterior cerebral artery with a dilated serpiginous vascular channel in the region of the vein of Galen which is further seen causing early opacification of the straight sinus in the arterial phase suggestive of dural arterio-venous fistula (Fig. 9A). Further there is prominence and early opacification of the torcula heterophili, left transverse sinus, left sigmoid sinus, and left internal jugular vein (Figs. 9B-9C).
Overall findings on CT & MRI were suggestive of diffuse cranio-facial arterio-venous malformation likely representing Cerebro-facial Arterio-venous Metameric Syndrome (CAMS). Cranial nerve examination was normal and tone was normal in all limbs. Patient was referred to Department of Ophthalmology for eye examination. Ophthalmologic examination revealed normal vision without any evidence of pathology. Therefore, based on characteristic distribution of lesion and site of involvement, diagnosis of Cerebro-facial arterio-venous Metameric syndrome type 1 & 2 Complex is made. Since the patient conceived, hence no intervention was given. Patient is kept on close follow up.
 |
Fig. 1 Extraoral photograph showing facial symmetry with diffuse swelling on left side of face. |
 |
Fig. 2 Intraoral photograph showing lesion on left buccal and adjacent labial mucosa (A), left pterygomandibular raphe region (B) and mid palatal region (C). |
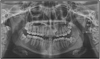 |
Fig. 3 Orthopantomograph (OPG) showing phleboliths (white arrow) along the anterior border of left ramus of mandible. |
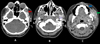 |
Fig. 4 Axial CT sections show bulky left temporalis (red arrow, A), medial and lateral pterygoid (purple arrow, B), masseter (green arrow, C), and buccinator (blue arrow, C) — with multiple dilated, serpiginous enhancing channels extending into the left temporal region, infratemporal fossa, masticator space, and parapharyngeal space. |
 |
Fig. 5 Non contrast axial (A to C) sections of CT through the face show phleboliths in the muscles of mastication on the left side. |
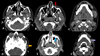 |
Fig. 6 CT angiography (arterial phase) shows dilated vessels in the left masticatory muscles with prominent draining veins (red arrow, B). Minimum intensity projection (D–F) highlights arterial supply from the left superficial temporal (yellow arrow, D), internal maxillary (light blue arrow, E), and facial arteries (purple arrow, F), branches of the left external carotid. |
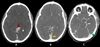 |
Fig. 7 Axial contrast-enhanced brain images show dilated vessels along left MCA and PCA territories, a large channel near the left medial thalamus (red arrow, A), early opacification of the straight sinus (yellow arrow, B), left transverse sinus (green arrow, C), sigmoid sinus, and internal jugular vein. Feeder from the left PCA (purple arrow, C). |
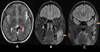 |
Fig. 8 Axial (A) and coronal (B, C) MRI show dilated serpiginous vessels at the internal cerebral vein and vein of Galen (red arrow, A) with T2 flow voids. Coronal FLAIR images (B, C) reveal left masticatory muscle hyperintensity (yellow arrows) with nodular hypointense foci. |
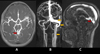 |
Fig. 9 MR angiography shows communication between a left PCA (P2) branch and a dilated vein of Galen channel (red arrow, A), with early straight sinus opacification, suggesting dural AV fistula. Early opacification of the torcula, left transverse sinus, sigmoid sinus, and internal jugular vein (yellow arrows, B and C) is also noted. |
Discussion
Bonet, Dechaume, and Blanc first recognized an association of vascular malformations involving the retina, face, and cerebrum in their report of two cases in 1937 [6,7]. This came to be known as Bonnet-Dechaume-Blanc syndrome or Wyburn-Mason syndrome. Later, Bhattacharya et al. in 2001 reported and reviewed this association with 15 case reports [1]. They suggested a disorder of neural crest development, which results in the metameric distribution of the lesion and the peculiar pattern of involvement at these sites. This came to be known subsequently as cerebrofacial arteriovenous syndrome (CAMS) [1]. CAMS is divided into three subgroups:
CAMS-1: Medial prosencephalic group, which involves the hypothalamus, hypophysis, and nose.
CAMS-2: Lateral prosencephalic group, which involves the retina, optic tract, thalamus, occipital lobe, and maxilla.
CAMS-3: Rhombencephalic group, which involves the pons, cerebellum, and mandible [1,8].
The present case is a complex of Types 1 and 2, which has been explained in the literature by the fact that mixed phenotypic expression can occur due to excessive insult to the prosencephalon at an early stage of development, resulting in overlapping territories [9].
Through limited published case reports and series, patients with CAMS present at a much younger age compared to those with sporadic arteriovenous malformations (AVMs); however, no specific sex predilection is noted [1,10]. The patient in this case had lesions since the age of 13, but due to the asymptomatic nature of the lesion, she did not seek professional advice. The clinical features vary depending on the involved metamere and its distribution. It has been noted that the most common symptoms patients present with include visual loss, neurologic deficits, and sometimes seizures. Facial vascular malformations are rarely reported, with some cases showing epistaxis and AVMs involving the nose.
The literature shows involvement of the retina in most cases; however, in six reported cases, there were no retinal AVMs [11,12]. In the present case, there is no involvement of the retina. Facial AVMs have been reported in a few cases in the literature. Bhattacharya et al., in their series of 15 cases, reported facial AVMs in only four cases [1]. The lesions generally remain silent or present as small, stable red angiomas from childhood and may later show growth during adolescence, becoming clinically relevant and symptomatic when they cause gingival bleeding and facial asymmetry. In a case report by Agid et al., there was involvement of the mandible and cheek, and in another case by Kang HS et al., the authors reported involvement of the tongue [3,5]. In the present case, there is facial asymmetry with involvement of the cheek, palate, and muscles of mastication, which is a rare finding. Additionally, there is evidence of calcifications representing phleboliths in the muscles of mastication, which has not been previously reported in the literature for CAMS. Cerebral AVMs are considered the most common in CAMS and typically present in a contiguous scattered manner but may sometimes occur as isolated lesions [1]. Typically, intracranial AVMs are described as clusters of smoke-like, small fine nidi with intervening normal brain tissue and the optic pathway, with a transdural arterial supply in some cases [9,13]. There are no reports of large dilated draining veins in cases of AVMs in the literature [13]. However, in the present case, cluster or nidus-like lesions are not seen, and a less common appearance, such as dilated draining veins, is evident with involvement of the dural sinuses. Cerebral lesions are localized to one hemisphere and are known for being multifocal and continuous in nature. These lesions remain clinically asymptomatic until discovery or may present with acute symptoms such as intracranial bleeding or progressive neurological deficits.
A detailed clinical examination, including physical, ophthalmological, neurological, and oral assessments, should be conducted. Imaging findings of AVMs depend on the size of the lesion and the presence or absence of calcification. CT is useful in determining the location and extent of the lesion as well as its proximity to adjacent structures [14]. This is particularly important in the case of intracranial lesions. In addition to CT, MR angiography is useful for demonstrating draining veins and feeder arteries [14]. Small lesions appear as iso- to hyperattenuating due to flowing blood within them, making them difficult to visualize on non-contrast CT. A “bag of worms” appearance is seen on MRI due to the presence of flow voids [14]. Cerebral angiography is performed to delineate the internal angioarchitecture and intranidal aneurysms [14,15]. The chief differential diagnosis includes cerebral proliferative angiopathy and cerebrofacial venous metameric syndrome such as Sturge Weber Syndrome [16,17]. CAMS can be differentiated from cerebral proliferative angiopathy by early draining veins seen in CAMS. CVMS are rarely associated with acute haemorrhage and cavernous malformations in comparison to CAMS [16,17].
Treatment varies depending on the sites involved and is individualized for each case. Most of the intracranial lesions are incurable; however, stereotactic radiosurgery, microsurgical resection and endovascular embolization are reserved for patients with severe symptoms [18]. Facial lesions can be managed with conservative therapies such as sclerotherapy.
Conclusion
This case highlights the wide spectrum of manifestations of cerebrofacial arteriovenous metameric syndrome, with vascular malformation involving the thalamus, dural sinuses, face, and muscles of mastication. CT angiography and MRI brain were helpful in diagnosing the condition, demonstrating feeder arteries, draining facial veins, bulky muscles of mastication, phleboliths, serpiginous vascular channels in facial spaces, and dilated dural sinuses. Since the phenotypic expression varies at the time of presentation, cerebral imaging is essential to rule out retinal and intracranial involvement in cases of facial AVMs, as these are prone to haemorrhage and life-threatening complications.
Funding
This article received no specific funding.
Conflicts of interest
The authors declare no conflicts of interest in regards to this article.
Data availability statement
All the data relevant to the publication are presented within the article.
Informed consent
Informed consent has been obtained from subject for inclusion of their details & images in manuscript.
References
- Bhattacharya JJ, Luo CB, Suh DC, Alvarez H, Rodesch G, Lasjaunias P. Wyburn-Mason or Bonnet-Duchaume-Blanc as cerebrofacial arteriovenous metameric syndrome (CAMS)—a new concept and new classification. Intervent Neuroradiol 2001;7:5–17. [Google Scholar]
- Wong IYC, Batista LL, Alvarez H, Lasjaunias PL. Craniofacial arteriovenous metameric syndrome (CAMS) 3- a transitional pattern between CAM 1 and 2 and spinal arteriovenous metameric syndromes. Neuroradiol 2003;45:611–615. [Google Scholar]
- Kang HS, Han MH, Kwon BJ, Yoon BW, Chang KH. Cerebellopontomandibular vascular malformation: a rare type of cerebrofacial arteriovenous metameric syndrome. J Neurosurg 2005;102:156–160. [Google Scholar]
- Brinjikji W, Nicholson P, Hilditch C, Krings T, Pereira V, Agid R. Cerebrofacial venous metameric syndrome- spectrum of imaging findings. Neuroradiol 2020;62(4):417–425. [Google Scholar]
- Agid R, Terbrugge KG. Cerebrofacial venous metameric syndrome 2 plus 3: facial and cerebral manifestations. Intervent Neuroradiol 2007;13:55–58. [Google Scholar]
- Bonnet P, Dechaume J, Blanc E. L'anevrisme cirsoide de la retine. (Anevrisme racemeux). Ses relations avec l'anevryme cirsoide du cerveau. Le Journal Medical de Lyon 1937;18:165–178. [Google Scholar]
- Wyburn-Mason R. Arteriovenous aneurysm of midbrain and retina, facial naevi and mental changes. Brain 1943;66:163–203. [Google Scholar]
- Ng JC, Appuhamy C, Lee W. Cerebrofacial arteriovenous metameric syndrome with hypopituitarism: a rare association. BMJ Case Reports 2018;2018:bcr–2017–222708. [Google Scholar]
- Couly G, Coltey P, Eichmann A, Le Douarin NM. The angiogenic potentials of the cephalic mesoderm and the origin of brain and head blood vessels. Mech Dev 1995;53:97–112. [Google Scholar]
- Jiarakongmun P, Alvarez A, Rodesch G, Lasjaunias P. Clinical Course and Angioarchitecture of Cerebrofacial Arteriovenous Metameric Syndrome. Interv Neuroradiol 2002;8:251–264. [Google Scholar]
- Ponce FA, Han PP, Spetzler RF. Associated arteriovenous malformation of the orbit and brain: a case of Wyburn-Mason syndrome without retinal involvement. Case report. J Neurosurg 2001;95:346–349. [Google Scholar]
- Gibo H, Watanabe N, Kobayashi S. Removal of an arteriovenous malformation in the optic chiasm. A case of Bonnet-Dechume-Bland syndrome without retinal involvement. Surg Neurol 1989;31:142–148. [Google Scholar]
- Willinsky RA, Lasjaunias P, Terbrugge K. Multiple cerebral arteriovenous malformations (AVMs): review of our experience from 203 patients with cerebral vascular lesions. Neuroradiology 1990;32:207–210. [Google Scholar]
- Smith AB. Vascular malformations of the brain: radiologic and pathologic correlation. J Am Osteopath Coll Radiol 2012;1(1):10–22. [Google Scholar]
- Kikuchi K, Kowada M, Sakamoto T, Tamakawa Y, Sakuragi S. Wyburn-Mason Syndrome: report of a rare case with computed tomography and angiographic evaluations. CT: J Comput Tomogr 1998;12:111–115. [Google Scholar]
- Larson AS, Brinjikji W, Krings T, Guerin JB. The cerebrofacial metameric syndromes: An embryological review and proposal of a novel classification scheme. Interv Neuroradiol. 2022;28(5):595–603. [Google Scholar]
- Lakhani DA, Khan M. Cerebrofacial arteriovenous metameric syndrome. Radiology 2024;313(2):e241156. [Google Scholar]
- O'Loughlin L, Groves ML, Miller NR, Pearl MS. Cerebrofacial Arteriovenous Metameric Syndrome (CAMS): a spectrum disorder of craniofacial vascular malformations. Childs Nerv Syst 2017;33:513–6. [Google Scholar]
Cite this article as: Gupta S, Chaudhary C, Gupta S, Aggarwal A, Ghosh S, Gupta S. 2025. Cerebro-facial arteriovenous metameric syndrome: report of a rare case with involvement of muscles of mastication. J Oral Med Oral Surg. 31, 24: https://doi.org/10.1051/mbcb/2025029
All Figures
 |
Fig. 1 Extraoral photograph showing facial symmetry with diffuse swelling on left side of face. |
| In the text | |
 |
Fig. 2 Intraoral photograph showing lesion on left buccal and adjacent labial mucosa (A), left pterygomandibular raphe region (B) and mid palatal region (C). |
| In the text | |
 |
Fig. 3 Orthopantomograph (OPG) showing phleboliths (white arrow) along the anterior border of left ramus of mandible. |
| In the text | |
 |
Fig. 4 Axial CT sections show bulky left temporalis (red arrow, A), medial and lateral pterygoid (purple arrow, B), masseter (green arrow, C), and buccinator (blue arrow, C) — with multiple dilated, serpiginous enhancing channels extending into the left temporal region, infratemporal fossa, masticator space, and parapharyngeal space. |
| In the text | |
 |
Fig. 5 Non contrast axial (A to C) sections of CT through the face show phleboliths in the muscles of mastication on the left side. |
| In the text | |
 |
Fig. 6 CT angiography (arterial phase) shows dilated vessels in the left masticatory muscles with prominent draining veins (red arrow, B). Minimum intensity projection (D–F) highlights arterial supply from the left superficial temporal (yellow arrow, D), internal maxillary (light blue arrow, E), and facial arteries (purple arrow, F), branches of the left external carotid. |
| In the text | |
 |
Fig. 7 Axial contrast-enhanced brain images show dilated vessels along left MCA and PCA territories, a large channel near the left medial thalamus (red arrow, A), early opacification of the straight sinus (yellow arrow, B), left transverse sinus (green arrow, C), sigmoid sinus, and internal jugular vein. Feeder from the left PCA (purple arrow, C). |
| In the text | |
 |
Fig. 8 Axial (A) and coronal (B, C) MRI show dilated serpiginous vessels at the internal cerebral vein and vein of Galen (red arrow, A) with T2 flow voids. Coronal FLAIR images (B, C) reveal left masticatory muscle hyperintensity (yellow arrows) with nodular hypointense foci. |
| In the text | |
 |
Fig. 9 MR angiography shows communication between a left PCA (P2) branch and a dilated vein of Galen channel (red arrow, A), with early straight sinus opacification, suggesting dural AV fistula. Early opacification of the torcula, left transverse sinus, sigmoid sinus, and internal jugular vein (yellow arrows, B and C) is also noted. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


