| Issue |
J Oral Med Oral Surg
Volume 31, Number 3, 2025
|
|
|---|---|---|
| Article Number | 23 | |
| Number of page(s) | 6 | |
| DOI | https://doi.org/10.1051/mbcb/2025027 | |
| Published online | 28 July 2025 | |
Case Report
Zygomatic implants for supporting nasal epitheses in large carcinological rhinectomies: case reports
1
Department of Oral and Maxillofacial Surgery and Stomatology, Roger Salengro Hospital, Lille University Hospital Centre, France
2
The International Association of Oral and Maxillofacial Medecine (AIMOM) is based in Villeneuve-d'Ascq, France
* Correspondence: marine.bovis@hotmail.fr
Received:
10
November
2024
Accepted:
21
May
2025
Two patients aged 55 and 62 underwent total rhinectomy followed by radiotherapy for the treatment of nasal squamous cell carcinoma. Initially, a conventional nasal epithesis was fitted, but was not tolerated due to allergic reactions to skin adhesives and stability issues caused by the weight of spectacles on the epitheses. A second surgical procedure was therefore performed to place two zygomatic implants, anchored at a distance from the irradiated bone, providing additional retentive support via a bar-and-clip connection. Post-implant follow-up revealed early failure of one of the two implants in the first patient, necessitating its replacement. A two-year follow-up confirmed the stability of all implants, with a notable improvement in prosthetic retention and patient comfort, alongside minimal complaints. This two-case report describes an innovative approach relying exclusively on the use of zygomatic implants to support a nasal prosthesis, a method still scarcely documented in the literature. It provides a detailed account of the surgical protocol followed and opens up promising prospects for the rehabilitation of post-oncological midfacial defects.
Key words: Dental implants / zygoma / radiotherapy / surgical oncology / maxillofacial prosthesis
© The authors, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The management of maxillary and facial defects resulting from tumour excision surgery poses a challenge for maxillofacial surgeons. Among these, class VI defects according to Brown et al. (2010), involving the naso-maxillary region, are considered some of the most difficult to treat [1].
For these complex cases, prosthetic obturation offers clear advantages, including immediate restoration of appearance and the possibility of monitoring the cavity. This epithesis can be retained using tissue adhesives or attached to spectacles; however, these methods have notable limitations. Its stability can be improved with bone-anchored retention devices. The placement of standard-length implants in the midfacial region remains the standard approach, with implants typically placed in the nasal floor and glabella, even in potentially irradiated bone [2–6]. Meanwhile, plate anchorage systems, such as epiplating, represent an interesting alternative for partial rhinectomies [7,8].
In cases of total or extended rhinectomies, the use of zygomatic implants (ZI) may provide a particularly reliable retention solution, positioned away from irradiated bone. Their efficacy is well documented for the rehabilitation of maxillary defects, with high survival rates (77–100%) with a low complication rate [9–11]. However, their exclusive use, in a horizontal position, to support nasal prostheses is a more recent concept. First reported by Bowden et al. [12] after conventional implant failure, this technique has yet to find its place in clinical practice. Available studies primarily concern two retrospective, single-centre studies [13,14]. The first, carried out by Scott et al., involved the rehabilitation of 28 patients using ZI [13]. The second, conducted in the Salisbury department, addressed the rehabilitation of 34 patients with bone-anchored implants, including 8 ZI [14].
Although these reports have provided valuable data, there is still a lack of detailed documentation on long-term outcomes, technical variations, and the adjustments required in different clinical contexts. By presenting recent cases with prolonged follow-up, this report aims to identify potential improvements and guide practitioners towards the optimal application of this innovative technique. Finally, the writing of this article followed the CARE guidelines [15], which are part of the EQUATOR network.
Case study
Observation and therapeutic decision
All clinical information and observations for both patients are summarised in Table I. The first patient complained of chronic epithesis mobility and was reluctant to undergo further surgery, so enhancing his existing prosthesis met his needs. In contrast, the second patient, who had developed extensive facial dermatitis due to tissue adhesives, was willing to consider reconstruction. However, given his ongoing smoking habit, reconstructive surgery seemed high-risk, making an improved epithesis the more suitable option. Consequently, both patients were offered an implant-retained epithesis. After reviewing the isodose curves (indicating less than 50Gy to the zygomatic bones) and assessing bone volume on CT scan, the decision was made to place two horizontally positioned ZI emerging at the level of the piriform rim. Both patients had been in remission for 27 and 17 months, respectively, so there was no contraindication to implant placement. Because patient1 underwent a less radical tumour resection, 15mm of nasal bone and the nasal septum were preserved.
Summary of each patient's characteristics.
Pre-operative assessment
Implant planning was carried out using 3D reconstruction software (DTX-Studio) to position two implants horizontally, ensuring that the zygomatic bone was fully engaged. The main objectives were to ensure parallelism between the two implants, as well as an emergence offset of 3 to 4 mm in height, to facilitate their placement during surgery. The department used the Zygoma Branemark system (Nobel Biocare, Kloten, Sweden), with a TiUnite or machined surface, a diameter of 3.9 mm, and a length ranging from 30 mm to 52.5 mm. Furthermore, a 3D craniofacial model was 3D-printed from the CT data using ITK-SNAP (v3.8.0, University of Pennsylvania, USA) and 3D Slicer (v4.11, Harvard Medical School, USA), then printed via a stereolithography resin printer (Fig. 1). On this physical set-up, pilot holes were created to simulate the implant drills, helping us validate implant axes and refine the intraoperative approach.
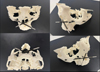 |
Fig. 1 Setup on a 3D model of the upper facial structure for patient 1. |
Operating protocol
Surgery was performed under general anaesthesia. Access was achieved via the nasal route, at the site of the pyramid amputation, and the incision was made at the junction of the mucosa and skin. A progressive subperiosteal dissection was carried out to identify the residual bony edges of the piriform orifice and to locate the emergence of the infraorbital nerve. The infraorbital rim and the zygomatic bone were exposed. A pilot drill (2.9 mm) was used to create the entry point in the medial wall of the maxillary sinus and to drill the zygomatic bone to the full length of the implant. The drilling was then widened to 3.5 mm to allow the insertion of the ZI (50 N/cm). Near-perfect parallelism was achieved between the two implants (Figs. 2–5), with the head of the implant inclined at 45° to the coronal plane. Healing abutments were placed to prevent soft tissue proliferation during the healing phase.
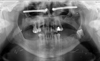 |
Fig. 2 Patient 1, Post-operative panoramic radiograph. |
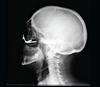 |
Fig. 3 Patient 1, post-operative teleradiograph, after maxillary edentation and placement of the prosthetic bar. |
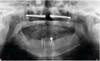 |
Fig. 4 Patient 2, post-operative panoramic radiograph. |
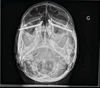 |
Fig. 5 Patient 2, post-operative frontal radiograph (Blondeau view). |
Prosthesis fabrication
The final prosthesis, made by an epithesist, was completed and fixed to the implants six months after surgery (Fig. 6), during which the patient wore a conventional epithesis. Impressions of the nasal cavity were taken using a custom tray with clip-on implant transfers. A Dolder-type gold bar was designed to connect the two implants and distribute stress. Circular in shape, this bar also incorporated a central vertical anti-rotational component to enhance stability and function as a stabilising tripod. Once passive adaptation of the bar was confirmed, it was attached to the epithesis by means of a retentive rider. This fixation system included an intermediate structure made of self-curing acrylic resin, firmly anchored to the silicone elastomer. The prosthesis was fabricated in appropriate shades, based on CT scan data and photographs taken before the onset of the disease. The follow-up, provided twice a year by the epithesist, aimed to maintain retention and make any necessary aesthetic adjustments.
 |
Fig. 6 Patient 2, Placement of the prosthetic bar 6 months after surgery, Intrados of the nasal epithesis, Nasal epithesis in place, clipped to the implant-supported bar. |
Post-operative follow-up
Patient 1 experienced an immediate failure of osseointegration, with mobility and rotation of the implant, as well as migration into the zygomatic space, visible on the CT scan. The implant was replaced with a new one, positioned further forward in the body of the zygoma. A few weeks later, maxillary sinusitis developed on the same side but was effectively treated with antibiotics, and the new implant integrated successfully. No complications were observed for patient 2. Issues with crusting and endonasal dryness, related to the absence of nocturnal use of the epithesis, were resolved with regular wear. Long-term implant follow-up was conducted separately from the oncological follow-up of the nasal cavity. Appointments took place every three months in the first year, every six months in the second year, and then annually. At the two-year follow-up, all implants demonstrated perfect osseointegration and excellent prosthetic retention. The results therefore showed an implant survival rate of 75% (3/4 implants), with one implant subsequently replaced successfully.
Discussion
The results of this study confirm the effectiveness of ZI in improving nasal epithesis retention following total or subtotal rhinectomies. These findings align with those of Scott et al. (overall survival rate of 98%) and Ethunandan (87%) [13,14]. In our two cases, despite one initial implant failure requiring replacement, the long-term outcomes were excellent. Nasal prosthetic rehabilitation was performed independently of oral rehabilitation, as both patients were satisfied with their removable maxillary prostheses. However, had implant-supported oral rehabilitation been considered, zygomatic implantation could have been an option depending on the severity of maxillary bone atrophy. In this context, the use of an epiplating system or conventional mid-facial implants could also have been discussed to support the nasal epithesis [7,8].
The implants were placed after radiotherapy, which was feasible because the cumulative dose to the zygoma was below 50 Gy [16]. Implants were placed after rhinectomy, as initial treatment was performed in another centre where bonded epithesis had been preferred. Several studies currently recommend placing ZI at the time of tumour resection to expedite prosthetic rehabilitation and reduce the risks of invasive surgery in irradiated areas [4]. According to Butterworth et al., this approach has a 4% failure rate compared to 11% for secondary implants, although the difference is not significant [10]. Scott et al. (Morriston experience), placed 56 ZI (46%) prior to radiotherapy, and reported only one failure, emphasizing the advantages of early implantation [13]. However, our two implant surgeries were performed within the recommended one-year post-radiotherapy period, which has been associated with significantly improved implant survival rates [4,16,17].
In oncological resection, nasal bones are preserved as far as possible to provide a robust tissue base for the prosthesis and allow the nasal bridge to support glasses. This was not possible for the second patient; depending on the connection system, a glabellar implant could have been considered [14,18]. Nevertheless, Scott et al., who rehabilitated 28 patients using ZI, opted for an additional nasal implant whenever the nasal cavity width allowed. In their work, Scott and Ethunandan used guided surgery (stereolithographic or chrome-cobalt guide), manufactured in two parts and resting on the opposing piriform rim, to ensure accurate implant positioning [13,14]. Recently, modified oncology ZI (Southern Implants, Nobel) have been developed, featuring a polished midsection and a 15 mm apical thread to minimize debris accumulation and facilitate cleaning. In Boyes-Varley's study, on post-maxillectomy defects, they achieved a 100% survival rate [19]. However, Butterworth's study showed these implants doubled the failure risk (HR = 0.56) compared to conventional ZI [10], indicating further research is needed to assess their equivalence.
The literature shows considerable diversity in protocols and techniques for loading ZI. For “classic” maxillary atrophy, immediate loading is generally preferred. In oncologic post-maxillectomy contexts, most studies recommend 2–6 months of healing [9]. According to Scott et al.—who reported a 98% implant survival rate—the prosthesis was fabricated and loaded only six weeks postoperatively, though this early loading involved primary implantation, differing from our approach [13]. By comparison, Butterworth recommends an average loading period of 9.3 months for secondary implantations in combined facial defects [10]. We chose a shorter six-month interval, during which the implants were not subjected to mechanical stress. Ethunandan's study proposed a different protocol depending on the extent of the rhinectomy [14].
In both our cases, a bar retention system was used, and no failures were observed after loading. This bar, with its central vertical element, avoided the need for an additional implant in the nasal cavity while minimizing emergence-related stresses. However, Scott et al. preferred magnets, and according to Ethunandan, bar retention was associated with a higher failure rate than magnets (33% vs. 9%, p = 0.0564). These data suggest that while a bar system offers specific advantages, magnets may present a lower risk of failure [13,14].
Managing complications, such as the initial osseointegration failure seen in one of our patients, raises questions about long-term success factors. These were examined in the retrospective study conducted in Salisbury, which found that variables such as age, sex, and tumour histology had no significant influence on implant failure rates. Although failures were more frequent in smokers (13%) than in non-smokers (7%), the difference was not statistically significant. However, the study did show, a correlation between implant location and failure rate: implants placed in the orbital and maxillary rims failed more often than those positioned in the zygoma, nasal floor, or glabella [14]. No specific study evaluates hyperbaric oxygen therapy (HBO) for the survival of ZI after radiotherapy, but Granström et al. reported a significant improvement in extra-oral implant survival in irradiated patients treated with HBO [20]. Based on this Swedish experience, Abu-Serriah et al. proposed HBO before implant placement in irradiated periorbital sites, given its positive impact on irradiated bone, particularly in the absence of other therapeutic alternatives [3]. Mechanical stresses laterally, or the “lever arm” effect, are significantly greater than those on standard dental implants, where occlusal force is usually parallel to the implant axis. These stresses may explain failures in patients who have undergone extensive maxillectomy [13]. In mid-facial defects, however, the main forces involve nasal epithesis retention rather than occlusal loads, potentially accounting for the very low failure rate post-loading, as seen in the Morriston series by Scott et al., where only one failure occurred out of 56 zygomatic implants. Our own results, with long-term survival of all four implants — including one successfully replaced — are consistent with these findings.
Conclusion
Zygomatic implants offer a promising alternative to conventional implants for nasal prosthetic rehabilitation, thanks to their length, which ensures strong bone engagement and promotes osseointegration. Their positioning, often outside the irradiated zone, may reduce failure risk. Although postoperative complications such as osseointegration failure, sinusitis, or facial paraesthesia may occur, as illustrated by the manageable complications observed in our two cases, these did not compromise the overall effectiveness of the rehabilitation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors.
Conflicts of interest
The authors declare that they have no conflicts of interest related to this study. No external funding, grants, or financial support were received for the conception, execution, or publication of this research. The authors alone are responsible for the content and writing of this article.
Data availability statement
All data analysed in this study were obtained through university access or interlibrary loan. All sources used are appropriately cited in the manuscript.
Author contribution statement
M. Bovis assisted with the surgical procedures, collected the clinical data, and wrote the manuscript. G. Raoul performed the surgeries and critically revised the manuscript. Both authors approved the final version.
Informed consent
Written informed consent was obtained from both patients for the publication of their anonymised data and medical images.
References
- Brown JS, Shaw RJ. Reconstruction of the maxilla and midface: introducing a new classification. Lancet Oncol 2010;11:1001–1008. [Google Scholar]
- Wolfaardt J, Gehl G, Farmand M, et al. Indications and methods of care for aspects of extraoral osseointegration. Int J Oral Maxillofac Surg 2003;32:124–131. [Google Scholar]
- Abu-Serriah MM, McGowan DA, Moos KF, et al. Outcome of extra-oral craniofacial endosseous implants. Br J Oral Maxillofac Surg 2001;39:269–275. [Google Scholar]
- Abu-Serriah MM, McGowan DA, Moos KF, et al. Extra-oral endosseous craniofacial implants: current status and future developments. Int J Oral Maxillofac Surg 2003;32:452–458. [Google Scholar]
- Flood TR, Russell K. Reconstruction of nasal defects with implant-retained nasal prostheses. Br J Oral Maxillofac Surg 1998;36:341–345. [Google Scholar]
- Nishimura RD, Roumanas E, Moy PK, et al. Nasal defects and osseointegrated implants: UCLA experience. J Prosthet Dent 1996;76:597–602. [Google Scholar]
- Nowak SM, Silk H, Ilankovan V. New approach for the anchorage of nasal implants without osseointegration using the Epiplating system for a magnetic prosthesis. Br J Oral Maxillofac Surg 2020;58:488–490. [Google Scholar]
- Davies I, Himesha B. Medawela RMS, Evans PL, et al. Immediate Prosthetic nasal rehabilitation following rhinectomy: indications and limitations of the epiplating system − a case series. Br J Oral Maxillofac Surg 2025; S0266435625000105. [Google Scholar]
- Hackett S, El‐Wazani B, Butterworth C. Zygomatic implant‐based rehabilitation for patients with maxillary and mid‐facial oncology defects: a review. Oral Dis 2021;27:27–41. [Google Scholar]
- Butterworth CJ. Primary vs secondary zygomatic implant placement in patients with head and neck cancer—A 10-year prospective study. Head Neck 2019;41:1687–1695. [Google Scholar]
- Pineau M, Nicot R, Lauwers L, et al. Das Zygoma-Implantat in unserer täglichen Praxis: Teil I: Behandlungsplan und OP-Technik. SWISS Dent J SSO − Sci Clin Top 2018;128:701–705. [Google Scholar]
- Bowden JR, Flood TR, Downie IP. Zygomaticus implants for retention of nasal prostheses after rhinectomy. Br J Oral Maxillofac Surg 2006;44:54–56. [Google Scholar]
- Scott N, Kittur MA, Evans PL, et al. The use of zygomatic implants for the retention of nasal prosthesis following rhinectomy: the Morriston experience. Int J Oral Maxillofac Surg 2016;45:1044–1048. [Google Scholar]
- Ethunandan M, Downie I, Flood T. Implant-retained nasal prosthesis for reconstruction of large rhinectomy defects: the Salisbury experience. Int J Oral Maxillofac Surg 2010;39:343–349. [Google Scholar]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep 2013; bcr 2013201554. [Google Scholar]
- Koudougou C, Bertin H, Lecaplain B, et al. Postimplantation radiation therapy in head and neck cancer patients: literature review. Head Neck 2020;42:794–802. [Google Scholar]
- Claudy MP, Miguens Jr SAQ, Celeste RK, et al. Time interval after radiotherapy and dental implant failure: systematic review of observational studies and meta-analysis. Clin Implant Dent Relat Res 2015;17:402–411. [Google Scholar]
- Wälivaara D-Å, Isaksson S, Johansson L-Å. Frontal bone and modified zygomatic implants for retention of a nasal prosthesis: surgical planning using a three-dimensional computer software program. J Plast Surg Hand Surg 2011;45:109–112. [Google Scholar]
- Boyes-Varley JG, Howes DG, Davidge-Pitts KD, et al. A protocol for maxillary reconstruction following oncology resection using zygomatic implants. Int J Prosthodont 2007;20:521–531. [Google Scholar]
- Granström G, Tjellström A, Brånemark PI. Osseointegrated implants in irradiated bone: a case-controlled study using adjunctive hyperbaric oxygen therapy. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg 1999;57:493–499. [Google Scholar]
Cite this article as: Bovis M, Raoul G. 2025. Zygomatic implants for supporting nasal epitheses in large carcinological rhinectomies: case reports. J Oral Med Oral Surg. 31, 23: https://doi.org/10.1051/mbcb/2025027
All Tables
All Figures
 |
Fig. 1 Setup on a 3D model of the upper facial structure for patient 1. |
| In the text | |
 |
Fig. 2 Patient 1, Post-operative panoramic radiograph. |
| In the text | |
 |
Fig. 3 Patient 1, post-operative teleradiograph, after maxillary edentation and placement of the prosthetic bar. |
| In the text | |
 |
Fig. 4 Patient 2, post-operative panoramic radiograph. |
| In the text | |
 |
Fig. 5 Patient 2, post-operative frontal radiograph (Blondeau view). |
| In the text | |
 |
Fig. 6 Patient 2, Placement of the prosthetic bar 6 months after surgery, Intrados of the nasal epithesis, Nasal epithesis in place, clipped to the implant-supported bar. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


