| Issue |
J Oral Med Oral Surg
Volume 29, Number 3, 2023
|
|
|---|---|---|
| Article Number | 36 | |
| Number of page(s) | 4 | |
| DOI | https://doi.org/10.1051/mbcb/2023036 | |
| Published online | 20 December 2023 | |
Case Report
A curious swelling of the lingual gingiva: case report
1
Institut de Médecine Bucco-Dentaire du CHU de Nice, 28 boulevard de Riquier 06300 Nice
2
Faculté de Chirurgie Dentaire, Université Côte d'Azur, EA 7354 MICORALIS (Microbiologie Orale, Immunothérapie et Santé), 06357 Nice, France
3
Laboratoire Central d'Anatomie et Cytologie Pathologiques (LCAP), 30 avenue de la Voie Romaine, Nice
* Correspondence : Strokov.s@chu-nice.fr
Received:
24
November
2023
Accepted:
26
November
2023
Introduction: Melanocytic nevi are benign tumors composed of nevus cell, thought to be derived from the neural crest. While they are unusual in the oral mucosa, they contrastingly represent the most diagnosed pigmented lesion on the skin. Observations: A 68-year-old woman presented with an asymptomatic ”epulis-like”, pale pink swelling of the lingual gingiva and slowly growing in the last 6 months. Excisional biospy was performed with a hypothesis of fibroma but the histopathologic examination revealed surprisingly an achromic intramucosal nevus. Conclusion: The unusual appearance in these cases suggests that oral melanocytic nevi should be included as a rare possibility in the differential diagnosis of swellings in the gingiva, regardless of their clinical pigmentation.
Key words: unpigmented nevus / melanocytic lesion / gingival tumor / oral cavity
© The authors, 2023
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Melanocytic nevus is a benign proliferation of nevus cells either in the epithelium or in the subepithelial stroma that can be congenital or develop later. Intraoral lesions are uncommon, and the etiology and pathogenesis are poorly understood. The origin of nevus cells has been postulated to be from cells that migrate from the neural crest to the epithelium and submucosa, or to be from altered resident melanocytes [1]. They exist as extremely diversified forms, such as rounded, linear, zonal, figured, metameric and multiple nevi [2].
Microscopically, several subtypes are recognized, classification is dependent on the location of nevus cells. Intramucosal nevus was the most common type (61%), followed by common blue nevus (23%), compound nevus (7%), and junctional nevus (3%). The hard palate was the most commonly affected site (33%), followed by the buccal mucosa (18%), vermilion border of the lip (18%), and gingiva (16%) [3,4]. Women account for 60% of patients [5]. Nevi can display different intensities of pigmentation, they can be brown, bluish gray or almost black but 13% may be non-pigmented and reddish [5]. Melanocytic nevi are sometimes macular, but usually slightly raised. Malignant transformation of an oral benign melanocytic nevus is highly improbable [1,6].
Case report
A 68-year-old Caucasian woman consulted for the presence of a painless, pinkish swelling of the gingiva. The lesion was situated at the mandible, on the lingual side of the premolar and molar region (teeth 34 to 37). The patient did not remember the presence of this lesion, the evolution time was unknown but she reported that it had been noticeable for approximately 6 months. On intraoral examination, the lesion was linear, sessile, well circumscribed, measured 0,8 cm × 2 cm and raised above the surface by approximately 0,4 cm. It was normal in color, firm in consistency and it clinically seemed to be a fibroma (Fig. 1).
An excisional biopsy was performed and microscopic analysis showed a proliferation of melanocytes cells in the connective tissue (Figs. 2A-2D). We noticed no junctional activity. A fibrous connective tissue zone separates the nevus cell theques from the overlying epithelium. It shows an architectural and cytological maturation gradient and spreads to the deep chorion. The superficial cells are round, medium-sized, with regular monomorphous nuclei. They show a tendency to cluster into theques (nests) but don't show intracellular melanin (Fig. 2B). The amount of pigment was minimal and could be detected only in isolated nevus cells situated in the middle zone. These cells have less cytoplasm, and resemble like lymphocytes (Fig. 2C). Deeper nevus cells appear elongated and spindle shaped and assume a neuroid or fibroblastic appearance (Fig. 2D). There was no mitotic figure.
The nevus cells were immunohistochemically positive for S-100 protein (Fig. 3A), Melan-A (Fig. 3B) and HMB-45 (Fig. 3C). Intra-cellular melanin was present only in a group of nevus cells from the middle zone. The final diagnosis is a non-pigmented intramucous nevus of oral cavity.
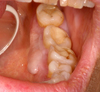 |
Fig. 1 Intra-oral photograph of case 1 shows a linear, sessile, well circumscribed lesion on the lingual aspect of the mandibular gingiva. |
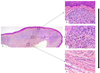 |
Fig. 2 Photomicrograph of case 2 shows an intradermal nevus at low magnification (Fig. 3A, HE × 40). The architectural and cytological maturation gradient was evident at higher magnification with superficial nevus cells in theque formation without intracellular melanin (3B, HES × 100), cells in the middle zone, with only melanin in some isolated nevus cells (3C, HES × 100) and deeper nevus cells appear elongated and spindle shaped without intracellular melanin (3D, HES × 100). |
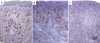 |
Fig. 3 Immunohistochemical features (×100): 4A − Antibody anti PS100, 4B − Melana, 4C − Antibody anti-HMB45. |
Discussion
We present a clinical achromic melanocytic nevus case located to gingival mucosa. Intra-oral melanocytic nevi are relatively rare lesions, represented only 0,1% [7] of all accessioned biopsies, or less (0,067%) [4]. Intra-oral nevi may occur at any age, but more than 50% of patients are between the second and third decade and affected more often female than man, similar to this case [8]. Unlike for their cutaneous counterparts, the different histological subtypes of oral melanocytic nevi don't have any distinguishing clinical characteristics, and can't be differentiated from a malignant melanoma. However, they are usually asymptomatic, solitary, well circumscribed, less than 1 cm, macular or nodular in appearance, and brown or blue in color [9,10].
As these lesions were asymptomatics, they are usually discovered during routine intraoral examination. Our case was atypical for the big size (0,8 × 2,5 cm), and presents an uncommon form as a hyperplasia swelling.
In a serie of 191 nevi of the oral cavity, Buchner and Hansen, showed that intramucous nevi are the most frequent type and found that 13% of the cases was non pigmented nevi [11]. However, this percentage may be underestimating since clinical unpigmented lesions are less likely to be biopsied [8,9,12]. It's important to remind that 15% of all histological subtypes of oral nevi may not show any clinical pigmentation and may be misdiagnosed as other non-melanocytic swelling of oral mucosa [13,14].
Their surface can be smooth, rough, papillomatous, or warty and can be confused with other lesions. [8,9,11]. The most common diagnoses for the nonpigmented lesions were fibroma, papilloma and nevus. In Ferreira study's the most common clinical diagnosis for intramucosal nevi was nevus in 29,2% of the cases and fibroma was the sole diagnosis in 25% [4].
In our case the lesion was non-pigmented, pinkish a similar color with the surrounding mucosa and with a smooth surface. This case occurs on gingival which is not the most usual site which affected the palate, gingiva is the 4th common site.
In intramucosal nevus, usually the superficial cells are larger and heavily pigmented, whereas the deeper cells have less cytoplasm and less pigment [12,15]. We found the contrary, masses of nevus cells devoid of pigment are beneath the surface epithelium, the only few pigmented cells were observed deeper. This finding may be correlate to the clinical presentation of this lesion, without any apparent pigmentation.
We found that nevus cells expressed melanocytic markers such as PS-100, HMB-45 and Melena. The cells expressed PS100 more intensely than HMB-45 like in the study of Gazit et al. [16] and Melana. PS-100 over-expression has been expected in melanotic tumor, and often associated to amount of melanin pigment, but in amelanotic nevi it has also been found [17]. The loss of protein HMB45 expression in deep dermis confirm the architectural gradient maturation in correlation with the previous morphological findings on histological sections stained by Hematoxylin-Eosin.
Clinical diagnosis of non-pigmented nevi is a challenge, because the nevus shows a pinkish color that is indistinguishable from that of the surrounding mucosa [18]. The differential diagnosis of this uncommon intra-oral nevus doesn't include the other pigmented lesions or melanoma but hyperplasia related with medication or familial hyperplasia and only histological diagnosis results to be conclusive [18].
Conclusion
This present case suggests that melanocytic lesion such as nevi or melanoma must be included in the differential diagnosis of gingival swelling, regardless of their clinical pigmentation.
Conflict of interest
The authors declares that they have no conflicts of interest in relation to this article.
Funding
This research did not receive any specific funding.
Ethical approval
Ethical approval was not required.
Informed consent
This article does not contain any studies involving human subjects.
Authors contributions
Svyat Strokov = Writing original draft / Hélène Raybaud = Writing original draft / Nathalie Cardot-Leccia = Conceptualization reviewing and editing / Christine Voha = Writing original draft.
References
- Regezi JA, Sciubba JJ, Jordan RCK. Oral pathology: clinical pathologic correlations. Saunders 2003, 448 p [Google Scholar]
- Patrick RJ, Fenske NA, Messina JL. Primary mucosal melanoma. J Am Acad Dermatol 2007;56:828–834. [CrossRef] [PubMed] [Google Scholar]
- Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. St. Louis, MO: Saunders 2009 382–388. [Google Scholar]
- Ferreira L, Jham B, Assi R, Readinger A, Kessler HP. Oral melanocytic nevi: a clinicopathologic study of 100 cases. Oral Surg Oral Med Oral Pathol Oral Radiol 2015;120:358–367. [CrossRef] [PubMed] [Google Scholar]
- Cawson R, Binnie WH, Barrett AW, Wright J. Oral disease. Mosby 3ed, 2001. [Google Scholar]
- Meleti M, Mooi WJ, Casparie MK, van der Waal I. Melanocytic nevi of the oral mucosadno evidence of increased risk for oral malignant melanoma: an analysis of 119 cases. Oral Oncol 2007;43:976–981. [CrossRef] [PubMed] [Google Scholar]
- Buchner A, Merrell PW, Carpenter WM. Relative frequency of solitary melanocytic lesions of the oral mucosa. J Oral Pathol Med 2004;33:550–557. [CrossRef] [PubMed] [Google Scholar]
- Buchner A, Leider AS, Merrell PW, Carpenter WM. Melanocytic nevi of the oral mucosa: a clinicopathologic study of 130 cases from northern California. J Oral Pathol Med 1990;19:197–201. [CrossRef] [PubMed] [Google Scholar]
- Buchner A, Hansen LS. Pigmented nevi of the oral mucosa: a clinicopathologic study of 36 new cases and review of 155 cases from the literature: Part I. A clinicopathologic study of 36 new cases. Oral Surg Oral Med Oral Pathol 1987 63:566–572. [CrossRef] [PubMed] [Google Scholar]
- Alfredo A, et al. Pigmented lesions of the oral mucosa. Burket's Oral Med 2021; n. pag. [Google Scholar]
- Buchner A, Hansen LS. Pigmented nevi of the oral mucosa: a clinicopathologic study of 36 new cases and review of 155 cases from the literature: Part II. Analysis of 191 cases. Oral Surg Oral Med Oral Pathol 1987;63:676–682. [CrossRef] [PubMed] [Google Scholar]
- Buchner A, Hansen LS. Pigmented nevi of the oral mucosa: a clinicopathologic study of 32 new cases and review of 75 cases from the literature. Part 1: A clinicopathologic study of 32 cases. Oral Surg Oral Med Oral Pathol 1979 48:131–142. [CrossRef] [PubMed] [Google Scholar]
- Muller S. Melanin-associated pigmented lesions of the oral mucosa: presentation, differential diagnosis, and treatment. Dermatol Ther. 201 2010. [Google Scholar]
- Alawi F. Pigmented lesions of the oral cavity: an update. Dent Clin N Am 2013;57:699–710. [CrossRef] [Google Scholar]
- Freitas DA, Bonan PR, Sousa AA, Pereira MM, Oliveira SM, Jones KM. Intramucosal nevus in the oral cavity. J Contemp Dent Pract 2015;16:74–76. [CrossRef] [PubMed] [Google Scholar]
- Gazit D, Daniels TE. Oral melanocytic lesions: differences in expression of HMB-45 and S-100 antigens in round and spindle cells of malignant and benign lesions. J Oral Pathol Med 1994;23:60–64. [CrossRef] [PubMed] [Google Scholar]
- Nakajima T, Watanabe S, Sato Y, Kameya T, Shimosato Y, Ishihara U. Immunohistochemical demonstration of S100 protein in malignant melanoma and pigmented nevus, and its diagnostic application. Cancer 1982;50:912–918. [CrossRef] [PubMed] [Google Scholar]
- Porrini R, Valente G, Colombo E, Cannas M, Sabbatini. Non pigmented melanocytic nevus of the oral cavity : a case report with emphasis on the surgical excision procedures. Minerva Stomatol 2013;62:43–49. [PubMed] [Google Scholar]
All Figures
 |
Fig. 1 Intra-oral photograph of case 1 shows a linear, sessile, well circumscribed lesion on the lingual aspect of the mandibular gingiva. |
| In the text | |
 |
Fig. 2 Photomicrograph of case 2 shows an intradermal nevus at low magnification (Fig. 3A, HE × 40). The architectural and cytological maturation gradient was evident at higher magnification with superficial nevus cells in theque formation without intracellular melanin (3B, HES × 100), cells in the middle zone, with only melanin in some isolated nevus cells (3C, HES × 100) and deeper nevus cells appear elongated and spindle shaped without intracellular melanin (3D, HES × 100). |
| In the text | |
 |
Fig. 3 Immunohistochemical features (×100): 4A − Antibody anti PS100, 4B − Melana, 4C − Antibody anti-HMB45. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


