| Issue |
J Oral Med Oral Surg
Volume 25, Number 3, 2019
|
|
|---|---|---|
| Article Number | 28 | |
| Number of page(s) | 3 | |
| Section | Cas clinique et revue de la littérature / Up-to date review and case report | |
| DOI | https://doi.org/10.1051/mbcb/2019013 | |
| Published online | 22 August 2019 | |
Up-to Date Review And Case Report
Progressive facial hemiatrophy (Parry-Romberg syndrome): short case report
1
Faculté d'odontologie, Université Claude Bernard Lyon I, 11 rue Guillaume Paradin, 69008 Lyon, France
2
Unité tête et cou, chirurgie oncologique, CRLCC Léon Bérard, 28 promenade Lea et Napoleon Bullukian, 69008 Lyon, France
* Corresponding author: anne-gaelle.bodard@lyon.unicancer.fr
Received:
4
June
2019
Accepted:
29
June
2019
Introduction: The Parry Romberg syndrome (PRS) is a mosaic disease of unknown aetiology which mostly affects women. The facial hemiatrophy generally begins during the early childhood. It has a great impact on social life, and aesthetic rehabilitation is a major challenge. Observation: A 38 years-old-female patient presented with an enucleation of the left eye, due to multiple hamartomas and progressive facial hemiatrophy. The placement of 2 extraoral implants was proposed to bear an ocular epithesis. Comments: PRS develops between the 2nd and 20th year of life, and stabilizes at the adult age. Its main characteristic is a progressive facial hemiatrophy, which involves skin, fat tissues, muscles and osteocartilaginous tissues. 20% of patients have neurological symptoms, and epilepsy is often described as a side effect of the disease. Conclusion: Diagnosis of localized scleroderma has to be eliminated, as these 2 entities are very similar. Major facial reconstructive surgery is often proposed to restore aesthetics.
Key words: facial hemiatrophy / progressive / rehabilitation / follow up
© The authors, 2019
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The Parry Romberg syndrome (PRS) or progressive facial hemiatrophy has been described by Parry in 1825 and by Romberg in 1846 [1]. The main characteristic is a progressive unilateral facial atrophy of the skin of the maxillary region, subcutaneous tissue and sometimes the adjacent craniofacial bone. It mostly appears in female patients aged between 2 and 20. Neurologic involvement concerns 20% of the patients. This mosaic disease has a great impact on social life; aesthetic rehabilitation is being a major challenge for these patients' rehabilitation. This paper highlights the long-term effects of the syndrome and therapeutics proposals to overcome them.
Observation
A 38 years-old-female patient presented with an enucleation of the left eye. Her medical history began at birth with multiple benign tumors of the legs and the lacrymal foramen of the left eye. The karyotype was normal. Surgery was performed at the age of 5 months to remove the tumors. Histopathology revealed multiple hamartomas. Left endophtalmia and left progressive facial hemiatrophy were noticed from the age of 15 months. The differential diagnosis consisted of localized sclerodermitis. For this patient, the atrophy was more diffuse and affected the whole hemiface. Type I neurofibromatosis was also discussed but there was no NF1 gene mutation.
At 9 years old, the patient presented with evolutive epilepsia and brain tomodensitometry revealed large amounts of porencephaly in the left hemisphere. At 14 years old, a mandibular odontogenous fibroma led us to perform an interruptive mandibulectomy, mandibular reconstruction being achieved at the end of growth with a rib graft. Dental implants were placed on the graft to restore the dental arch.
Enucleation had then to be realized because of pain caused by major endophtalmia. Growth was highly affected, with an adult size of 1.32 m and major facial left atrophy. A mild mental retardation was noticed. Lipofillings were proposed to enhance facial contour.
The patient consulted at the age of 38 for the rehabilitation of her left eye with an epithesis. Clinical examination revealed facial asymmetry, decreased but sufficient bone volume on the periorbital left area, and hypodevelopment of the left hemiface. She also complained of frequent headaches.
A dental CT scan was performed to evaluate the bone amount for periocular implant placement. The CTscan also revealed an osteolysis of the sphenoid and a large hamartoma involving the soft tissues (Fig. 1).
Two extra oral implants of 4 mm length were placed under general anaesthesia to bear an oculo-palpebral epithesis (Fig. 2). After 4 months for osseointegration, the epithesis was made.
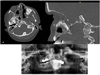 |
Fig. 1 (A and B) CT scan showing multiple intracranial hamartomas; (C) orthopantomography showing the hamartomas and the intraoral status. |
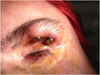 |
Fig. 2 Extraoral implants for the bearing of the epithesis. |
Commentaries
This case report was first published when the patient was aged 17 [2]. Republishing this case 20 years after allows a long term follow up of the case, which is of particular interest in slowly progressive diseases such as PRS. The PRS is a rare disease, mostly affecting female patients from the age of 2 years old [3]. It develops between the 2th and 20th year of life, and stabilizes at the adult age. Late stabilization of the disease (up to 75 years) has been rarely described [4]. Various aetiologies have been proposed, of which autoimmune dysfunction [5] on the same spectrum as localized scleroderma. Serum autoantibodies, inflammatory histopathology and positive response to immunosuppressors are found. Another hypothesis is a modified migration of the neural crest cells, intracranial vascular malformation or neurotrophic viral infection [5]. Patients with early onset seem to be more severely affected, with more severe aesthetic and functional deficits [6]. This patient presented with tumors related to PRS at birth, which is early and could corroborate embryogenic ectodermic aetiology of the disease [7]. It often affects the maxillary and orbital region [4,8]. 20% of patients have neurological symptoms, such as headaches and cognitive impairment, brain atrophy, epilepsy, or hemiplegia [4,9]. In this case report, the patient presented with headaches and epilepsy which severity increased with the patient's growth.
Ocular involvement concerns 16% of PRS patients [4,10–12]. In this case report, eye involvement was early and led to enucleation of the left eye.
Alopecia is sometimes described [9]. Some authors have discussed the premium effect of soft tissues retraction on bone growth and asymmetry, but it seems that bone atrophy is also a primary phenomenon rather than a process secondary to soft tissue retraction [13].
Differential diagnosis consisting of localized scleroderma must be eliminated, as it also affects young patients. Clinical presentation includes ophthalmic and neurological complications, with a facial atrophy described as “en coup de sabre”, and a loss of skin elasticity [4]. Due to the overlapping clinical presentations of these 2 diseases, some authors consider them as clinical variants. It also has been shown that conversion of localized scleroderma to PRS has been reported, and that 30–40% patients with PRS present with typical scleroderma changes [4]. Lipoatrophy (also described as Barraquer-Simons syndrome) has also to be eliminated: the lipoatrophy is systemic and not limited to the face, and there are complement levels abnormalities and C3 seric nephritic factor [14,15]. Nonetheless, these 2 diseases are also strongly associated [16].
PRS shows various types of imaging features, and a CT scan must be performed to evaluate intracranial and facial involvement. Nonetheless, the incidence and cause of these alterations remain unknown [4,17].
Considering intra-oral status, previously to the mandibular reconstruction, the patient reported delayed teeth eruption, which is another characteristic of the PRS [5].
A few cases of bilateral PRS syndrome have been described [3,14]. It affects 2–7% of cases [17].
As facial aesthetic is a main complaint, plastic surgical procedures are often proposed. Periocular lipofilling performed before the realisation of the epithesis allowed a better esthetical result. This surgery must be performed after disease stabilization [5]. Autologous fat grafting seems to show favourable results for soft tissues deficiency [5], as well as hyaluronic acid filling [14]. For severe diseases, free tissue transfer is preferable [5].
Conclusion
PRS is a clinical diagnosis supported by imaging and histopathology. Multimodal follow up (rheumatology, neurology, maxillofacial surgery, oral surgery, and prosthodontics) must be performed. Psychological and social problems must also be considered [6].
Conflicts of interest
The authors declare that they have no conflicts of interest in relation to this article.
References
- Romberg MH, Hennoch EH. Kranheiten des nervensystems (IV: Trophoneurosen). Klinische Ergebnisse, Berlin: Verlag Albert Förstner 1846;75–81. [Google Scholar]
- Derex L, Isnard H, Revol M. Progressive facial hemiatrophy with multiple benign tumors and hamartomas. Neuropediatrics 1995;26:306–309. [CrossRef] [PubMed] [Google Scholar]
- Tkachenko E, Cunningham MJ, O'Donnell PJ, Levin NA. Adult-onset bilateral Parry-Romberg syndrome. JAAD Case Rep 2019;5:209–212. [CrossRef] [PubMed] [Google Scholar]
- Tollefson MM, Witman PM. En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol 2007;56:257–263. [CrossRef] [PubMed] [Google Scholar]
- Schulz KP, Dong E, Truong TA, Maricevich RS. Parry-Romberg syndrome. Clin Plast Surg 2019;46:231–237. [CrossRef] [PubMed] [Google Scholar]
- Kumar NG, Maurya BS, Sudeep CS. Parry Romberg syndrome: literature review and report of three cases. J Maxillofac Oral Surg 2019;18:210–216. [CrossRef] [Google Scholar]
- El Khedy J, Abbas O, Rubeiz N. A review of Parry-Romberg syndrome. J Am Acad Dermatol 2012;67:769–284. [CrossRef] [PubMed] [Google Scholar]
- Tang XJ, Liu W, Yang B, Shi L, Yin L, Zhang ZY. Parry-Romberg syndrome with rare maxillofacial deformities: a report on two cases. J Cranio Maxillofac Surg 2014;42:780–783. [CrossRef] [Google Scholar]
- Kuah CY, Koleva E, Gan JJL, Iqbal T. Parry-Romberg syndrome in patient with scleroderma. BMJ Case Rep 2018;14. [Google Scholar]
- Orozco-Covarrubias L, Guzman-Meza A, Ridaura-Sanz C, Carrasco Daza D, Sosa-de-Martinez C, Ruiz-Maldonado R. Scleroderma “en-coup-de-sabre” and progressive facial hemiatrophy. Is it possible to differentiate them? J Eur Acad Dermatol Venereol 2002;16:361–366. [CrossRef] [PubMed] [Google Scholar]
- Tolkachjov SN, Patel NG, Tollefson MM. Progressive hemifacial atrophy: a review. Orphanet J Rare Dis 2015;10:39. [CrossRef] [PubMed] [Google Scholar]
- Auvinet C, Glacet-Bernard A, Coscas G, Cornelis P, Cadot M, Meyringnac C. Parry-Romberg progressive facial hemiatrophy and localized scleroderma. Nosologic and pathogenic problems. J Fr Ophtalmo 1989;12:169–173. [Google Scholar]
- Hennocq Q, Facchini A, Kverneland B, Bodemer C, Picard A, Khonsari RH. Craniofacial bone atrophy in Parry Romberg syndrome demonstrated by using a Bayesian hierarchical model. J Craniomaxillofac Surg 2019;8. [Google Scholar]
- Watchmaker J, Saadeh D, Lam C, Vashi NA. A case of bilateral Parry-Romberg syndrome successfully treated with hyaluronic acid filler augmentation. J Cosmet Dermatol 2019;15. [PubMed] [Google Scholar]
- Doolittle DA, Lehman VT, Schwartz KM, Wong-Kisiel LC, Lehman JS, Tollefson MM. CNS imaging findings associated with Parry-Romberg syndrome and en coup de sabre: correlation to dermatologic and neurologic abnormalities. Neuroradiology 2015; 57:21–34. [CrossRef] [Google Scholar]
- Ruhin B, Bennaceur S, Verecke F, Louafi S, Seddiki B, Ferri J. Progressive hemifacial atrophy in the young patient: physiopathology hypotheses, diagnosis and therapy. Rev Stomatol Chir Maxillofac 2000;101:287–297. [PubMed] [Google Scholar]
- Stone J. Parry-Romberg syndrome: a global survey of 205 patients using the Internet. Neurology 2003;61:674–676. [CrossRef] [Google Scholar]
All Figures
 |
Fig. 1 (A and B) CT scan showing multiple intracranial hamartomas; (C) orthopantomography showing the hamartomas and the intraoral status. |
| In the text | |
 |
Fig. 2 Extraoral implants for the bearing of the epithesis. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


