| Issue |
J Oral Med Oral Surg
Volume 26, Number 4, 2020
|
|
|---|---|---|
| Article Number | 42 | |
| Number of page(s) | 4 | |
| Section | Revue de la littérature / Literature review | |
| DOI | https://doi.org/10.1051/mbcb/2020021 | |
| Published online | 31 August 2020 | |
Literature Review
Pterygoid hamulus syndrome: a case report
1
Oral Surgery Department, University of Bordeaux, France
2
Oral Surgery Department, University Paris Descartes, France
* Correspondence: paul.galvez@u-bordeaux.fr
Received:
31
October
2019
Accepted:
26
May
2020
Introduction: Pterygoid hamulus syndrome (PHS) is a little-known differential diagnosis of orofacial pain. It is characterized by oropharyngeal pain, secondary to inflammatory bursitis of the tensor veli muscle of post-traumatic origin, frequently fostered by an associated hypertrophy of the hamular process. Observation: A 64-year-old female patient, type 2 diabetic, consulted for constant posterior palatal pain located near to 17, lasting for 10 years. The inspection did not reveal any mucosal lesions. Right hamulus palpation increased the pain and revealed hamulus hypertrophy. A diagnosis of PHS was evoked. Comment: A review of the literature is proposed. The treatment of PHS is initially conservative, but a surgical treatment can be proposed in case of morphological anomalies. Conclusion: PHS is a little-known syndrome whose diagnosis must be mentioned by the oral surgeon faced with chronic oropharyngeal pain. The diagnosis is clinical and radiological, the treatment is medical and/or surgical.
Key words: orofacial pain / pterygoid hamulus syndrome / hamulus hypertrophy
© The authors, 2020
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Misunderstood orofacial pains can lead to diagnostic errors and the performance of iatrogenic procedures. Pterygoid hamulus syndrome (PHS) is a little-known differential diagnosis of orofacial pain, causing significant diagnostic issues. Formalized by Hjorting-Hansen in 1987 [1], it is characterized by oropharyngeal pain localized in the soft palate, aggravated by the velar function and radiating to the pharynx and ear. It would be associated with inflammation of the bursal tendon of the palate tensor muscle (hamular bursitis), whose origin is post-traumatic, most often promoted by hypertrophy of the pterygoid hamulus facilitating intraoral trauma of the bursa.
This article reports a case of PHS supplemented by a literature review on this poorly known syndrome.
Observation
A 64-year-old patient with type 2 diabetes treated with insulin glargine and repaglinide consulted for constant posterior palatal pain in relation with 17. The pain had been present for 10 years. Pain was rated 9 out of 10 on a numerical scale. The inspection did not reveal any mucosal lesions. Palpation of the hamulus region on the right side revealed a prominent bony process. The continuous pressure in this region reproduced the symptomatology described by the patient and increased the pain. The remaining extraoral and intraoral examination revealed no other abnormalities. The pain did not evolve into seizures but was well localized and exacerbated by continuous pressure, which led the diagnosis to pain due to an excess of nociception. Their precise location in the hamulus region ruled out Ernest's syndrome (stylomandibular) and led us to suspect PHS. A computed tomography (CT) scan of the facial mass revealed an elongation of the medial and lateral blades of the pterygoid processes, more marked on the right side with a 7.2 mm length hamulus, compatible with PHS (Figs. 1 and 2). Conservative treatment with corticosteroid infiltration (triamcinolone acetonide 40 mg/mL, suspension for injection) was initiated. A re-evaluation was initiated every 3 months, combined with an injection based on the patient's reported symptomatology. Clinical follow-up of 18 months was performed after the first injection. A new injection was performed after 3 months, 9 months, and 15 months. Triamcinolone acetonide injections allowed a significant reduction in pain intensity (greater than 40% with a pain rating of 5 out of 10 on a numerical scale). The medical treatment was well-tolerated by the patient and she was not treated surgically.
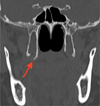 |
Fig. 1 Hypertrophy of the pterygoid hamulus (red arrow) on the computerized tomography − scan (coronal section). |
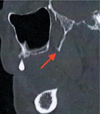 |
Fig. 2 Hypertrophy of the pterygoid hamulus (red arrow) on the computerized tomography − scan (para-sagittal section). |
Comments
The pterygoid hamulus is a hook-shaped bone process localized at the lower end of the medial pterygoid processes of the sphenoid bone. It is medially bypassed by the tendon of the tensor veli palatini muscle that connects to the fascia of the veli. This tendon is covered with a bursa, whose main function is to decrease the friction exerted on the hamular process by the tendon of the tensor muscle of the veli palatini muscle when moving [2].
Inflammation of this bursa (hamular bursitis) is believed to cause referred pain in the orofacial region that may mimic temporomandibular dysfunction, trigeminal or glossopharyngeal neuralgia, Eagle syndrome, local infection, or otitis [3].
Thirteen publications of PHS cases were retrieved [1,4–11,16,18–20]. The course of PHS patient care is characterized by medical nomadism and diagnostic wandering often leading to the performance of iatrogenic (dental treatments) and ineffective (persistence of chronic pain for several years) procedures. In the presented case, the diagnostic wandering lasted for 10 years.
The elements that lead to positive diagnosis of PHS are a characteristic disease history with diagnostic wandering and a clinical examination reproducing pain upon palpation of the pterygoid hamulus region. Sasaki et al. found that this pathology affected men in 79.3% of cases, with a median age of patients being 38.4 years [16]. Symptoms reported in PHS are pain, burning, or tension in relation to pterygoid hamulus. They are spontaneous or revealed by pressure and palpation. A projection of pain at the palatal level, toward the maxilla, toward the oropharynx, and oropharynx or ear may appear. These pains can cause functional discomfort during swallowing or eating [10]. Erythema or mucosal ulceration can be found. When the hamulus is hypertrophied, its bony hook is palpable. The triggering of pain (reproducing the patient's symptomatology) while applying continuous pressure for few seconds in the hamulus region is highly indicative of this diagnosis, [12]. Local infiltration of analgesics allows rapid pain relief [2].
The diagnosis can be confirmed by additional examinations such as a CT scan of the facial mass without injection, which will show hypertrophy of the hamulus, a fracture of the hamulus, or osteophytes [2]. Different etiologies (alone or associated) have been proposed to explain hamulus pain [11]: hamulus hypertrophy or elongation [5,6], bursitis or spasms of the tensor veli palatini muscle [2,10], hamulus osteophytes, and/or repeated trauma [6]. A mechanical conflict during closure encountered in the cases of posterior maxillary and mandibular edentulism, as well as wearing a removable maxillary prosthesis may be promoting factors [2].
Differential diagnoses of PHS are multiple: Ernest's syndrome (stylomandibular), glosso-pharyngeal neuralgia, trigeminal neuralgia, oropharyngeal tumors, stomatitis, otitis media, developmental accidents of the third maxillary molars, and Eagle syndrome (stylohyoid) [13]. PHS can also be confused with temporomandibular dysfunction: A retrospective cohort analysis showed that PHS was present in 20% of patients consulting for temporomandibular dysfunction (92/464 patients) [14].
Different studies have calculated the average size of the hamulus. Eyrich et al. calculated the length of the hamulus to be 5 mm in the left and 4.9 mm in the right [11]. According to Putz et al., the average length of the hamulus is 7.2 mm [15]. A study of 29 people showed an average length of the hamulus of to 6.8 ± 1.4 mm [16]. More recently, Orhan et al. found a hamulus length of 5.48 ± 1.94 mm on the left and 5.40 ± 2.00 mm on the right after a retrospective analysis of 396 cone beam computed tomography scans [17]. There is no threshold to affirm the hypertrophy of the hamulus, but according to the literature data, its average length is approximately 5–7 mm and a value greater than 8 mm can be considered larger than usual.
The pain encountered in PHS was initially attributed to a mechanical conflict between the crown and the enlarged hamulus during oral closure in a patient with posterior edentulism [4]. Sasaki et al. reported fibrosis or bursitis of the tensor veli palatini muscle by excessive pressure of the tendon fascia and explained the projected pains by the hypothesis of associated nervous hyperactivity of the large and small palatal nerves, the glossopharyngeal nerve, and facial nerve [16]. In addition, Charberneau et al. explained the presence of pain in the absence of hamulus hypertrophy according to three anatomical features: a lower position of the medial pterygoid, a higher position of the palatal mucosa, and a fine palatal mucosa [7].
There is currently no consensus on the treatment of PHS. Initially, Sasaki et al. emphasized patient therapeutic education recommending to protect the hamulus area from mechanical irritations [16]. Initially, conservative treatment is implemented, combining the elimination of irritative factors with a limitation of repeated trauma (by adjusting removable dentures for example). Initial medical treatment consists of administering corticosteroids into the hamulus region, as in the present case. In addition, treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) per os is prescribed [2]. A reassessment is performed every 2 weeks, with medical treatment being continued until pain is reduced. This treatment is usually effective and sufficient. However, in case of long-term ineffectiveness, surgical treatment may be offered. It consists of an intraoral dissection of the hamulus under local anesthesia (Fig. 3). Some authors advocate first-line surgical treatment in case of hamulus hypertrophy, particularly in the presence of osteophytes or post-traumatic vicious calluses [2,13].
 |
Fig. 3 Hamulotomy in the treatment of a case of PHS refractory to medical treatment (case not presented). (A) Careful dissection of the right hamular region. (B) Removal of the tendon bursa of the tensor veli palatini muscle (being careful not to damage the muscle or twigs of the little palatine nerve). (C) Bone resection of the residual hamulus (until the whole palpable prominence disappears). It is worth noting the secretion of the ipsilateral accessory salivary glands (due to the stimulation of the nearby parasympathetic secretory nerve fibres during the surgical procedure). |
Conclusion
Misunderstood posterior palatal pains can lead to diagnostic errors and the performance of iatrogenic procedures. PHS is a little-known cause of orofacial pain. Diagnosis of PHS is based on the characteristic disease history, clinical examination via palpation of the pterygoid hamulus region that reproduces the patient's pain, and radiological confirmation in case of anatomical abnormalities. Initially, the treatment should be conservative (local infiltration of corticosteroids and NSAIDs per os), then surgical treatment may be offered secondarily in case of associated anatomical abnormalities.
Conflicts of interests
The authors declare that they have no conflicts of interest in relation to the publication of this article.
References
- Hjorting-Hansen E, Lous I. The pterygoid hamulus syndrome. Ugeskr-Laeger 1987;149:979–982. [Google Scholar]
- Shankland II WE. Pterygoid hamulus bursitis: one cause of craniofacial pain. J Prosth Dent 1996;75:205–210. [CrossRef] [Google Scholar]
- Ramirez LM, Sandoval GP, Ballesteros LE. Hamular bursitis and its possible craniofacial referred symptomatology: two case reports. Med Oral Patol Oral Cir Bucal. 2006;11:E329–E333. [Google Scholar]
- Gores RJ. Pain due to long hamular process in the edentulous patient. Lancet 1964;84:353–354. [Google Scholar]
- Hertz RS. Pain resulting from elongated pterygoid hamulus: report of case. J Oral Surg 1968;26:209–210. [PubMed] [Google Scholar]
- Wooten JW, Tarsitano JJ, Reavis DK. The pterygoid hamulus: a possible source for swelling, erythema, and pain: report of three cases. J Am Dent Assoc 1970;81:688–690. [CrossRef] [PubMed] [Google Scholar]
- Charbeneau TD, Blanton PL. The pterygoid hamulus: A consideration in the diagnosis of posterior palatal lesions. Oral Surg Oral Med Oral Pathol 1981;52:574–576. [Google Scholar]
- Brook IM. Pterygoid hamulus hyperawareness. Br Dent J 1982;153:150. [CrossRef] [PubMed] [Google Scholar]
- Hjorting-Hansen E, Lous I. Hamulus pterygoid syndrome. Tandlaegebladet 1987;91:833–837. [PubMed] [Google Scholar]
- Kronman JH, Padamsee M, Norris LH. Bursitis of the tensor veli palatini muscle with an osteophyte on the pterygoid hamulus. Oral Surg Oral Med Oral Pathol 1991;71:420–422. [Google Scholar]
- Eyrich GK, Locher MC, Warnke T, Sailer HF. The pterygoid hamulus as a pain-inducing factor: A report of a case and a radiographic study. Int J Oral Maxillofac Surg 1997;26:275–277. [CrossRef] [PubMed] [Google Scholar]
- Okeson JP. Management of TMD and occlusion, 2e ed., St Louis: CV Mosby; 1989:226. [Google Scholar]
- Bouguila J, Khonsari RH, Pierrefeu A, Corre P. Le syndrome d'Eagle: une douleur mal connue et mal reconnue ! Rev Stomatol Chir Maxillofac 2011;112:348–352. [Google Scholar]
- DuPont Jr JS, Brown CE. Comorbidity of pterygoid hamular area pain and TMD. Cranio 2007;25:172–176. [CrossRef] [PubMed] [Google Scholar]
- Putz P, Kroyer A. Functional morphology of the pterygoid hamulus. Anat Anz 1999;181:85–88. [CrossRef] [PubMed] [Google Scholar]
- Sasaki T, Imai Y, Fujibayashi T. A case of elongated pterygoid hamulus syndrome. Oral Dis 2001;7:131–133. [CrossRef] [PubMed] [Google Scholar]
- Orhan K, Sakul BU, Oz U, Bilecenoglu B. Evaluation of the pterygoid hamulus morphology using cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:48–55. [Google Scholar]
- Shankland WE 2nd. Bursitis of the hamular process. Part II: Diagnosis, treatment and report of three case studies. Cranio 1996;14:306–311. [CrossRef] [PubMed] [Google Scholar]
- Cho JY, Cheon KY, Shin DW, Chun WB, Lee H. Pterygoid hamulus bursitis as a cause of craniofacial pain: a case report. J Korean Assoc Oral Maxillofac Surg. 2013;39:134–138. [PubMed] [Google Scholar]
- Shetty SS, Shetty P, Shah PK, Nambiar J, Agarwal N. Pterygoid hamular bursitis: a possible link to craniofacial pain. Case Rep Surg. 2018;2018:5108920. [PubMed] [Google Scholar]
All Figures
 |
Fig. 1 Hypertrophy of the pterygoid hamulus (red arrow) on the computerized tomography − scan (coronal section). |
| In the text | |
 |
Fig. 2 Hypertrophy of the pterygoid hamulus (red arrow) on the computerized tomography − scan (para-sagittal section). |
| In the text | |
 |
Fig. 3 Hamulotomy in the treatment of a case of PHS refractory to medical treatment (case not presented). (A) Careful dissection of the right hamular region. (B) Removal of the tendon bursa of the tensor veli palatini muscle (being careful not to damage the muscle or twigs of the little palatine nerve). (C) Bone resection of the residual hamulus (until the whole palpable prominence disappears). It is worth noting the secretion of the ipsilateral accessory salivary glands (due to the stimulation of the nearby parasympathetic secretory nerve fibres during the surgical procedure). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


