| Issue |
J Oral Med Oral Surg
Volume 31, Number 3, 2025
|
|
|---|---|---|
| Article Number | 19 | |
| Number of page(s) | 9 | |
| DOI | https://doi.org/10.1051/mbcb/2025016 | |
| Published online | 24 June 2025 | |
Case Report
Sickle cell disease and jawbone osteomyelitis: case report and literature review
1
Henri Mondor Hospital, Dental Department, Paris-Cité University, Ile-de France, France
2
Laboratory of Molecular Oral Pathophysiology, INSERM 1138, Paris, France
* Correspondence: remi.durand@etu.u-paris.fr
Received:
14
November
2024
Accepted:
17
March
2025
Introduction: Sickle cell disease (SCD) can manifest with vaso-occlusive crises (VOC) in various anatomical locations. In this case, a mandibular osteomyelitis developed following a VOC but was initially misdiagnosed due to a lack of specific clinical and radiological indicators. This case report is accompanied by a brief review that highlights potential warning signs shared among similar cases, with the aim of aiding early identification and diagnosis. Observation: A 22-year-old male with SCD of Congolese origin was initially referred to the dental service at Henri Mondor Hospital for facial cellulitis during his hospitalization for a hip vaso-occlusive crisis (VOC). Due to the absence of specific clinical and radiological findings, a dental origin was initially suspected. However, as the patient's condition deteriorated, a diagnosis of mandibular osteomyelitis was eventually established. In addition to antibiotic therapy, a sequestrectomy was performed under general anaesthesia. Delayed healing was observed. Conclusion: Diagnosis osteomyelitis of jawbone in SCD patients can be challenging, as clinical and radiological signs may not always be evident. Dental practitioners and oral surgeons should be vigilant when managing these patients, as complications like mandibular osteomyelitis may present similarly to dental conditions but require prompt investigation and intervention to prevent serious health consequences.
Key words: Anemia / sickle cell / osteomyelitis / mandible / case reports
© The authors, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Sickle cell disease (SCD) is one of the most common genetic disorders, predominantly affecting individuals of Afro-Caribbean descent. In France, it stands as the most prevalent genetic disorder, currently affecting approximately 26,000 individuals, with around 600 children born with SCD each year [1]. This disorder, inherited in an autosomal recessive manner, is characterised by the production of an abnormal form of hemoglobin known as hemoglobin S (HbS) [2]. In environments with low oxygen levels, HbS molecules undergo polymerization into fibres, causing deformation of red blood cells, rendering them rigid and adhesive. This aggregation leads to hemolysis, resulting in hemolytic anemia [3]. Additionally, it can cause vascular occlusion in small blood vessels, reducing oxygen supply to affected organs and triggering painful vaso-occlusive crises (VOC).
These crises frequently manifest in bone marrow, likely due to marrow hypercellularity, which impedes blood flow, leading to regional hypoxia and subsequent tissue infarction. The affected bone marrow becomes more susceptible to infection, which can lead to osteomyelitis. Although osteomyelitis can affect any hematopoietically active bone marrow, the long bones of the extremities, such as the humerus, tibia, and femur, are most commonly affected with SCD patients facing a lifetime risk of 29–31% [4]. However, jaw osteomyelitis is rare among SCD patients, with an incidence rate of 3–5% [5].
This article presents a case of mandibular osteomyelitis in a patient with SCD, initially misdiagnosed due to a lack of specific clinical and radiological evidence. The case report is accompanied by a brief literature review aiming to identify potential warning signs shared among various cases reported.
Observation
A 22-year-old male of Congolese origin with G6PD-deficient sickle cell disease (SS type) was admitted to Henri Mondor Hospital (Créteil, France) with acute left mandibular cellulitis, two weeks after treatment for a vaso-occlusive hip crisis that required exchange transfusions.
His medical history included frequent hospitalizations for vaso-occlusive crises, episodes of splenic sequestration leading to a splenectomy in 2013, and a cholecystectomy in 2010. His regular treatment included folic acid and hydroxyurea.
In the emergency department, treatment with amoxicillin/clavulanic acid (6g/750mg per day) was initiated following a positive bacteriological sample for Staphylococcus aureus. The patient was then referred to the oral medicine department at Henri Mondor Hospital.
Extraoral examination revealed diffuse, soft swelling in left submandibular and buccal spaces with concomitant numbness along the distribution of the inferior alveolar nerve (IAN). Pericoronitis of a partially erupted and decayed lower third molar was identified as the cause of the cellulitis. This hypothesis was supported by findings of lesions at the apex of tooth 38 on the panoramic radiograph and cervico-facial scanner performed in the emergency department (Fig. 1). Antibiotic therapy was changed to amoxicillin 3g/day with Metronidazole 1.5g/day, leading to an improvement in infectious symptoms and a reduction in swelling at the 7-day check-up. Given the initial symptoms (cellulitis, sensitivity disturbance), a CBCT could have been indicated; however, the avulsion of the 38 was carried out with radiological support from the panoramic radiograph and cervico-facial scanner alone.
During extraction of tooth 38 under local anaesthetic, unexpected mobility of tooth 37 was detected, unobserved during the initial clinical and radiological examination. This necessitated its simultaneous extraction. Curettage of the alveolar bone revealed avascular necrotic tissue with loss of gingival attachment. An exploratory flap and curettage of the necrotic bone were performed at the surgical site. A specimen sent for histopathological analysis confirmed osteonecrosis with Staphylococcus aureus present. Antibiotic therapy was therefore continued to cover the mucosal healing period.
At the 7-day follow-up, a significant delay in healing was noted, with bone exposure and paleness of the covering tissues. Despite the absence of radiological evidence, left mandibular osteomyelitis was suspected, potentially related to vascular occlusion following the recent vaso-occlusive hip crisis.
One month follow-up revealed delayed healing with necrotic bone exposure from the retro-molar trigone to the lingual cortex of tooth 36, as well as significant mobility of teeth 36 and 35 (Fig. 2). Cone beam computed tomography (CBCT) revealed an ill-defined radiolucency extending horizontally from the extraction site of tooth 38 to the mesial root of tooth 36 and vertically to the upper wall of the mandibular canal, with loss of bone trabeculation (Fig. 3). Due to the risk of bone fracture, a sequestrectomy and extraction of non-conservable teeth (36/27) was performed, in collaboration with the maxillofacial surgery department, under general anaesthesia with exchange transfusion prior to the surgery.
Delayed mucosal healing was observed at both 15 day and 1.5 months post-operatively. At the 6 months follow-up, complete mucosal healing was observed (Fig. 2) but CBCT showed the persistence of a significant vertical bone defect after healing (Fig. 4). The restorative treatment of teeth 17, 16, 47, 46, 25 and the endondontic treatment of tooth 35 were carried out under nitrous oxide inhalation and local anaesthesia without vasoconstrictor to minimise the risk of triggering a new local vaso-occlusive crisis.
 |
Fig. 1 Initial orthopantomogram showing no bone changes in the left molar mandibular region. However, tooth 38 showed deep decay and periapical radiolucency. |
 |
Fig. 2 Intraoral photographs of affected site A) Pre-sequestrectomy (tooth 36) B) 6 months post-op. |
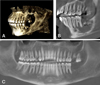 |
Fig. 3 Cone Beam Computed Tomography (CBCT) post-extraction (38, 37) and minimal curettage of necrotic alveolar bone at 1 month post-operative. A) 3D reconstruction of mandibular defect B) Sagittal section showing radiolucent necrotic bone, loss of trabecular structure distal to tooth 36C) Panoramic reconstruction showing “PDL thickening (35, 36), evidence of mobility, as well as the mottled, poorly defined necrotic zone distal to these teeth. |
 |
Fig. 4 CBCT at 6 month post-operative. A) 3D reconstruction showing the healed bone defect B) Sagittal section showing satisfactory reossification, with a residual vertical defect C) Panoramic reconstruction showing healing in sector 3, extraction sites 15, 27, 28, 36. Endo (35), restorations (25, 46) were subsequently carried out. |
Discussion
The literature has documented only 43 cases of jawbone involvement in SCD patients since 1971 (Tab. I). Most cases involved male patients, with a sex ratio of 36:7, indicating a higher male prevalence (84%). These These infections commonly affect young individuals, as in the present case (aged 22), with most patients occurring in the third decade (ages range between 6-40 years). These patients were primarily affected by homozygous S/S sickle cell anemia, which is the most common and severe form of SCD. Moreover, most cases were reported after a recent vaso-occlusive crisis (within the subsequent month) in patients who regularly suffer from VOC.
Chekroun et al. [25] reported that during a VOC episode, the entanglement of sickled cells within the inferior alveolar artery can lead to blockage, infarction, and aseptic pulpal necrosis. These ischemic regions may subsequently become necrotic and infected, either via hematogenous spread or local invasion along the periodontal ligaments. Among documented cases, the mandible is the most frequently affected part of the jaw, particularly in the molar and angle region. Olaitan et al. [5] suggested that this may be because the mandibular molar region is supplied solely by the ipsilateral inferior alveolar artery and periosteum. The anterior region is less susceptible to ischemia during a VOC episode involving the inferior alveolar artery, as it receives additional blood supply from contralateral vessels. Only three cases of maxillary involving the molar region have been reported in the literature because of its richer vascularization and its ability to easily supply occluded vessels [13,15–22].
Shroyer et al. [14] presented two separate hypotheses to elucidate the pathophysiology of osteomyelitis in sickle cell patients. The first hypothesis posits that a sickling crisis might induce bony infarctions, subsequently leading to bacterial colonization via hematogenous spread or local dissemination from the periodontal region. The second hypothesis suggests that a systemic infection could trigger a VOC, consequently causing bony infarction.
Initial symptoms may include facial pain and swelling, pus discharge, necrosis, periodontal issues such as tooth mobility, or numbness in the perioral region (predominantly situated in the lower lip in instances of inferior alveolar nerve involvement) (Tab. I). In the current case study, the patient initially experienced paresthesia of the lower lip and a dental infection in the molar region, associated with pericoronitis of the decayed lower third molar. Olaitan et al. [5] and Shroyer et al. [14] have also documented pericoronitis as a common cause of osteomyelitis among SCA patients with mandibular osteomyelitis.
The challenge in our case was the absence of radiological signs suggestive of bone involvement. Although numerous authors describe recognizable radiolucent or radiopaque lesions in affected areas, the initial CT scan and panoramic radiograph showed no abnormalities, except for minor lesions at the apices of tooth 38. Kavadia-Tsatala et al. [18] described radiopaque lesions in regions of vaso-occlusive events and infarctions. The lesions are characterised by decalcification surrounded by reactive sclerosis, followed by the formation of sclerotic bone that may be separated from the cortex by a thin radiolucent area, resulting in a bone-within-bone appearance. Conversely, osteomyelitis lesions may present as periosteal thickening, lytic lesions, osteoporosis, loss of trabecular architecture, sequestration, or new bone formation. Some authors as Shroyer et al. [14] or Watanabe et al. [19] have recommended the use of advanced radiological techniques, such as MRI, positron emission tomography or technetium Tc 99m scintigraphy to identify necrosis/ bone infract in cases of suspicion.
Regarding the pathogens involved, Al-Ismaili et al. [22] indicated that Salmonella is frequently responsible for long bone osteomyelitis among SCD patients, while Staphylococcus aureus is predominantly associated with jaw osteomyelitis, as seen in the present case. Other germs, such as Streptococcus spp., Pseudomonas spp., Actinomyces spp., and various mixed species, have also been identified as potential causative agents [5,11–13,14,16,22].
Early detection and timely referral to a specialised centre for expert management are crucial for these patients. Therefore, dental practitioners and oral surgeons should maintain a high level of suspicion for this condition when treating SCD patients.
As indicated in Table I, primary treatment typically includes antibiotic therapy, guided by microbiological and histopathological analyses of samples obtained from the necrotic area. However, Daramola et al. [9] suggested that antibiotics should be used alongside surgical interventions, as using them alone may not effectively manage the condition. The antibiotics most frequently employed are penicillin, metronidazole, and lincomycin.
The surgical approach varied depending on the extent of sequestration, ranging from simple curettage of the necrotic bone with tooth extraction to more extensive procedures like hemimandibulectomy with microvascular free flap reconstruction, as detailed in the study by Chang et al. [24].
Regarding anaesthesia management, some authors such as Al-Ismaili et al. [22] or Olaitan et al. [5] recommended general anaesthesia with a prior transfusion exchange to ensure optimal resection access. However, other researchers like Chekroun et al. [25] suggested using local anaesthesia with conscious sedation to avoid systemic complications while minimising procedural stress.
General anaesthesia is not indicated in patients with sickle cell disease and should be discussed with the patient's medical team on a case-by-case basis [26]. In our case, the recent vaso-occlusive crisis and the need to perform a thorough curettage with a possible risk of fracture given the extensive limits of the necrosis led us to perform the operation under general anaesthesia in consultation with our colleagues in the maxillofacial surgery department.
In consultation with the haematologist and considering the high risk in our patient, the other dental procedures were carried out under conscious sedation to limit stress and with local anaesthetic without vasoconstrictor to avoid triggering a new VOC in an area that has already been affected.
However, patients with sickle cell disease are not considered to be at risk of infection (SFCO 2012 [27]), they may receive conservative care from general dentists without special precautions for conservative care. Depending on the severity of the sickle cell disease, only procedures involving a risk of haemorrhage require special advice from the haematologist (transfusions if necessary), as it is the vascular problems that can lead to the ‘infectious’ risk, and hospital treatment for these procedures is therefore indicated.
Futhermore, vasoconstrictors are not totally contraindicated in sickle cell patients for procedures outside the affected areas (oral surgery and conservative care) [26]. If used carefully, they can be used to achieve a more effective surgical silence, thus guaranteeing the best possible ‘anti-stress’ for these patients at risk of VOC. However, intra-septal and intra-ligamentary injections are not discouraged [28].
Additionally, Mahendran et al. [23] proposed a non-surgical approach involving the administration of oral bisphosphonates for chronic osteomyelitis. Although their use remains controversial within the dental community, the authors argued that the risk of developing medication-related osteonecrosis of the jaw from oral bisphosphonates typically occurs only after prolonged use exceeding four years, with an incidence remaining below 1%. In contrast, the average duration of bisphosphonate therapy in osteomyelitis cases is around 12 months. Moreover, clinical improvement is often observed shortly after initiation of treatment after starting treatment, suggesting that it may be advisable to stop treatment if symptoms persist beyond eight weeks.
Literature review of jawbone osteomyelitis in patient with sickle cell disease.
Conclusion
Dental practitioners and oral surgeons must remain vigilant when providing care to SCD patients. This case report highlights the importance of recognising facial cellulitis in sickle cell patients who frequently experience vaso-occlusive crisis, as they may be misdiagnosed as odontogenic infections when they may in fact represent jawbone infarction. Prompt investigation and treatment are crucial for these patients, s delayed intervention can result in serious complications.
Funding
The authors declare that this research did not receive any specific funding.
Conflicts of interest
The authors declare that they have no conflicts of interest in relation to this article.
Data availability statement
The data used for this case can be accessed by contacting the corresponding author, D.R..
Ethics approval
Ethical approval was not required for this study.
Informed consent
Written informed consent was obtained from the patient.
References
- Alfa Cissé O, Durand-Zaleski I, Pirenne F, Chillotti L, Bénard S, Galactéros F. Épidémiologie et fardeau de la drépanocytose en France: analyse en vie réelle des données de l'échantillon généraliste des bénéficiaires (EGB). Rev Med Interne 2022;43: 444–445. [CrossRef] [PubMed] [Google Scholar]
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376: 2018–2031. [CrossRef] [PubMed] [Google Scholar]
- Bartolucci P, Galactéros F. Clinical management of adult sickle-cell disease. Curr Opin Hematol 2012;19:149–155. [CrossRef] [PubMed] [Google Scholar]
- Nwadiaro HC, Ugwu BT, Legbo JN. Chronic osteomyelitis in patients with sickle cell disease. East Afr Med J 2000;77:23–26. [PubMed] [Google Scholar]
- Olaitan AA, Amuda JT, Adekeye EO. Osteomyelitis of the mandible in sickle cell disease. Br J Oral Maxillofac Surg 1997;35:190–192. [CrossRef] [PubMed] [Google Scholar]
- Ryan MD. Osteomyelitis associated with sickle-cell anemia: report of a case. Oral Surg Oral Med Oral Pathol 1971;31:754–759. [CrossRef] [PubMed] [Google Scholar]
- Walker RD, Schenck KL Jr. Infarct of the mandible in sickle cell anemia: report of case. J Am Dent Assoc 1973;87:661–664. [CrossRef] [PubMed] [Google Scholar]
- Girasole RV, Lyon ED. Sickle cell osteomyelitis of the mandible: report of three cases. J Oral Surg 1977;35:231–234. [PubMed] [Google Scholar]
- Daramola JO, Ajagbe HA. Chronic osteomyelitis of the mandible in adults: a clinical study of 34 cases. Br J Oral Surg 1982;20:58–62. [CrossRef] [PubMed] [Google Scholar]
- Hammersley N. Mandibular infarction occurring during a sickle cell crisis. Br J Oral Maxillofac Surg 1984;22:103–114. [CrossRef] [PubMed] [Google Scholar]
- Grodecki EZ, Friedman JM. Mandibular osteomyelitis secondary to infarcts associated with sickle cell anemia. Spec Care Dentist 1985;5:217–221. [CrossRef] [PubMed] [Google Scholar]
- Iwu CO. Osteomyelitis of the mandible in sickle cell homozygous patients in Nigeria. Br J Oral Maxillofac Surg 1989;27:429–434. [CrossRef] [PubMed] [Google Scholar]
- Patton LL, Brahim JS, Travis WD. Mandibular osteomyelitis in a patient with sickle cell anemia: report of case. J Am Dent Assoc 1990;121:602–604. [CrossRef] [Google Scholar]
- Shroyer JV 3rd, Lew D, Abreo F, Unhold GP. Osteomyelitis of the mandible as a result of sickle cell disease: report and literature review. Oral Surg Oral Med Oral Pathol 1991;72:25–28. [CrossRef] [PubMed] [Google Scholar]
- Bishop K, Briggs P, Kelleher M. Sickle cell disease: a diagnostic dilemma. Int Endod J 1995;28:297–302. [CrossRef] [PubMed] [Google Scholar]
- Podlesh SW, Boyden DK. Diagnosis of acute bone/bone marrow infarction of the mandible in sickle hemoglobinopathy. Report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;81:547–549. [CrossRef] [PubMed] [Google Scholar]
- Borle RM, Prasant MC, Badjate SJ, Patel IA. Sickle cell osteomyelitis of the maxilla: a case report. J Oral Maxillofac Surg 2001;59:1371–1373. [CrossRef] [PubMed] [Google Scholar]
- Kavadia-Tsatala S, Kolokytha O, Kaklamanos EG, Antoniades K, Chasapopoulou E. Mandibular lesions of vasoocclusive origin in sickle cell haemoglobinopathy. Odontology 2004;92:68–72. [CrossRef] [PubMed] [Google Scholar]
- Watanabe M, Saito N, Nadgir RN, Liao JH, Flower EN, Steinberg MH et al. Craniofacial bone infarcts in sickle cell disease: clinical and radiological manifestations. J Comput Assist Tomogr 2013;37:91–97. [CrossRef] [PubMed] [Google Scholar]
- DeBlieux TK, Jackson N, Jeyakumar A, Townsend JA, Naik BV. Facial swelling in a sickle cell patient. Pediatr Dent 2014;36:104–106. [PubMed] [Google Scholar]
- Araújo JP, Cadavid AM, Lemos CA, Trierveiler M, Alves FA. Bilateral mandibular osteomyelitis mimicking periapical cysts in a patient with sickle cell anemia. Autops Case Rep 2015;5:55–60. [CrossRef] [Google Scholar]
- Al-Ismaili H, Nasim O, Bakathir A. Jaw osteomyelitis as a complication of sickle cell anaemia in three Omani patients: case reports and literature review. Sultan Qaboos Univ Med J 2017;17:e93–e97. [CrossRef] [PubMed] [Google Scholar]
- Mahendran K, Wali R, Patel V. Sickle cell osteomyelitis: a novel approach and review of the literature. Oral Surg 2021;14:365–370. [CrossRef] [Google Scholar]
- Chang K, Bollig C, Sclaroff A, Pipkorn P. Free tissue transfer in a patient with haemoglobin S-beta-thalassaemia disease and mandibular osteomyelitis. Otolaryngol Head Neck Surg 2022;166:186–187. [CrossRef] [PubMed] [Google Scholar]
- Chekroun M, Chérifi H, Fournier B, Gaultier F, Sitbon IY, Ferré FC et al. Oral manifestations of sickle cell disease. Br Dent J 2019;226:27–31. [CrossRef] [PubMed] [Google Scholar]
- Soualem H, Mainassara S, Beenjelloun L, Chbicheb S. Oral manifestations and management of sickle cell disease: a literature review. Int J Surg 2022;5:84 [Google Scholar]
- Société Française de Chirurgie Orale. Prise en charge des foyers infectieux bucco-dentaires. Med Buccale Chir Buccale 2012;18:251–314. [CrossRef] [EDP Sciences] [Google Scholar]
- Yue H, Xu X, Liu Q, Li X, Jiang W, Hu B. Association between sickle cell disease and dental caries: a systematic review and meta-analysis. Hematol 2020;25:309–319. [CrossRef] [Google Scholar]
Cite this article as: Durand R, Gaudimier Z, Flottes Y, Gogly B, 2025. Sickle cell disease and jawbone osteomyelitis: case report and literature review. J Oral Med Oral Surg. 31, 19. https://doi.org/10.1051/mbcb/2025016
All Tables
All Figures
 |
Fig. 1 Initial orthopantomogram showing no bone changes in the left molar mandibular region. However, tooth 38 showed deep decay and periapical radiolucency. |
| In the text | |
 |
Fig. 2 Intraoral photographs of affected site A) Pre-sequestrectomy (tooth 36) B) 6 months post-op. |
| In the text | |
 |
Fig. 3 Cone Beam Computed Tomography (CBCT) post-extraction (38, 37) and minimal curettage of necrotic alveolar bone at 1 month post-operative. A) 3D reconstruction of mandibular defect B) Sagittal section showing radiolucent necrotic bone, loss of trabecular structure distal to tooth 36C) Panoramic reconstruction showing “PDL thickening (35, 36), evidence of mobility, as well as the mottled, poorly defined necrotic zone distal to these teeth. |
| In the text | |
 |
Fig. 4 CBCT at 6 month post-operative. A) 3D reconstruction showing the healed bone defect B) Sagittal section showing satisfactory reossification, with a residual vertical defect C) Panoramic reconstruction showing healing in sector 3, extraction sites 15, 27, 28, 36. Endo (35), restorations (25, 46) were subsequently carried out. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


