| Issue |
J Oral Med Oral Surg
Volume 25, Number 3, 2019
|
|
|---|---|---|
| Article Number | 32 | |
| Number of page(s) | 4 | |
| Section | Cas clinique / Short case report | |
| DOI | https://doi.org/10.1051/mbcb/2019019 | |
| Published online | 09 September 2019 | |
Short Case Report
Mandibular ameloblastic carcinoma: case report and literature review
1
Interne DES Chirurgie Orale, service de chirurgie maxillo-faciale et stomatologie, Centre de soins et de consultations dentaires Lyon, France
2
Praticien Hospitalier, service d'odontologie du Centre anticancer Léon Bérard, Lyon, France
3
Maitre de conférences des Universités − Praticien Hospitalier, unité fonctionnelle de chirurgie du service de consultations et traitements dentaires et Praticien Hospitalier, service d'odontologie du Centre anticancer Léon Bérard, Lyon, France
* Correspondence: Margaux_02@hotmail.fr
Received:
11
March
2019
Accepted:
26
July
2019
Introduction: Ameloblastic carcinoma is an extremely rare malignant odontogenic tumor with predominantly mandibular localization. In most cases, it is treated surgically. Observation: Here, we describe a case of ameloblastic carcinoma. The patient presented a large expansive mass on the ascending branch of the left mandible, which was ulcerated and communicating with the oral cavity. He refused the proposed surgical treatment after being informed of the risk of facial decomposition. After several years, due to progressive symptomatology, he received palliative radiotherapy of 60 Gy divided into 30 sessions. Local control of the disease was achieved. Discussion: The efficiency of radiotherapy for ameloblastic carcinoma remains controversial. Conclusion: Radiotherapy appears to be a second-line approach when surgery is not feasible for ameloblastic carcinoma treatment.
Key words: ameloblastoma / carcinoma / odontogenic tumors
© The authors, 2019
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Ameloblastic carcinoma is an exceptional malignant tumor mainly occurring in the mandible. It may be primary or secondary to the transformation of a pre-existing ameloblastoma. Its prognosis is reserved because of its local aggressiveness and risk of metastatic expansion. Precise diagnosis is based on anatomopathological examination. The most common treatment is surgical via complete resection, followed by adjuvant radiotherapy. Here, we present the case of a patient with primary ameloblastic carcinoma treated with palliative radiotherapy; therapeutic approaches to this pathology in the literature are reviewed.
Observation
First consultation
A 73-year-old patient presented for consultation following discovery of a swelling of the left horizontal branch of the mandible. His case history was type II diabetes, rhizomelic pseudo-rheumatoid arthritis, and coronary artery disease. He was surgically treated for herniated disc and triple bypass. He had no allergies or significant family history. He was a former smoker (40 packs per year). Treatment included clopidogrel (Plavix®) 75 mg, glucophage (Metformin®) 850 ezetimibe + simvastatin (Vytorin®), ziloric (Allopurinol®), manidipine (Iperten®), and ramipril (Triatec®). He was previously biopsied and diagnosed with primary ameloblastic carcinoma located on the ascending left mandibular branch (Fig. 1); extension examniation was negative at the locoregional level and at a distance. The surgical team recommended resection surgery: dissection with fibula free flap reconstruction. However, the patient, in the view of the surgical risks and disfigurement, refused the surgery without follow-up.
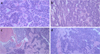 |
Fig. 1 Histological section of the biopsy showing cells with elongated nuclei assuming a palissadic appearance with clarified cells having an inverted nuclear polarity. The nuclei are the site of clear-cut nuclear atypia, and mitotic figures are visible. (a) Magnification ×50, (b) Magnification ×100, (c) Magnification ×200, (d) Magnification ×400. |
Consultation at 5 years
He was examined for increased swelling and complaint of episodes of local infections and frequent intraoral bleeding from tumors. On endobuccal examination, a lesion with 15-mm diameter was detected behind the last lower left molar, which was bleeding on contact, painless, ulcerated, and communicating with the oral cavity. The tongue, floor of the mouth, tonsillar lodge, and veil seemed to be unaffected. There was no restriction in mouth opening, hypoesthesia in the left labiomental area, involvement of cranial nerve pairs, or palpable lymphadenopathy. Panoramic radiography (Fig. 2) and imaging (Fig. 3) revealed an expansive bone lesion of the mandibular angle and left horizontal mandibular branch, invading the cortical layer, with gaseous content, and measuring 60 × 45 × 37 mm3. The lesion spared the condyle, parotid, and submaxillary gland. Extension examination revealed a single adenopathy of the ipsilateral facial pedicle, which was not very specific.
Surgery with reconstruction using microanastomosed fibula flap was proposed again, but after Multidisciplinary Consultative Meeting and given his arterial condition, radiotherapy for tumor control was proposed. The patient was again lost to follow-up.
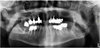 |
Fig. 2 Panoramic radiography showing diffuse osteolysis of the ascending branch of the left mandible 5 years after the first consultation. (Panoramic radiography showing diffuse osteolysis of the ascending branch of the left mandible 5 years after the first consultation.) |
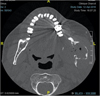 |
Fig. 3 Scan of facial bone window and in axial section at the mandibular level, at 5 years, showing an osteolytic and expansive lesion of the ascending branch of the left mandible. (Scan of facial bone window and in axial section at the mandibular level, at 5 years, showing an osteolytic and expansive lesion of the ascending branch of the left mandible.) |
Consolation at 6 years
One year later, magnetic resonance imaging (Fig. 4) revealed a larger lesion, measuring 75 × 55 × 54 mm3, near the base of the skull, in contact with the parotid gland and invading the masticatory space.
The patient's general condition was preserved. Clinical examination showed a clear progression of the tumor with increased face deformation. At the intraoral level, there was a large necrotic cavity behind the 36, which bled spontaneously.
After further discussion with the patient, he refused surgery (which corresponded to the reference treatment) once gain and requested “to stop bleeding.” Therefore, a conventional external radiotherapy for local and hemostatic control was proposed.
Radiotherapy was delivered 60 Gy divided in 30 sessions (2 Gy per fraction, 5 weekly fractions). At the end of irradiation, the patient reported excellent tolerance, despite known side effects, as well as disappearance of bleeding. Six months later, his general condition was preserved and he was asymptomatic. Clinical examination revealed improvements with mucosal healing, suggesting a single arch sequella. The patient only complained of hearing loss on the left side.
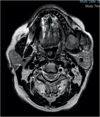 |
Fig. 4 T1 time MRI in axial section, at 6 years, through the mandible, showing the expansive process in relation to the ascending branch of the mandible. (T1 time MRI in axial section, at 6 years, through the mandible, showing the expansive process in relation to the ascending branch of the mandible.) |
Consolation at 7 years
One year after radiotherapy, follow-up imaging (Fig. 5) showed a relatively stable tumor (75 × 45 × 39 mm3), pushed back parotid without invasion, decreased local circumferential tissue component, and lack of ganglionic encroachment. A quarterly follow-up has been initiated.
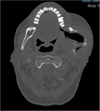 |
Fig. 5 Facial scan (bone window) in axial section 1 year after radiotherapy showing a stabilization of this expansive process and communication with the oral cavity. (Facial scan (bone window) in axial section 1 year after radiotherapy showing a stabilization of this expansive process and communication with the oral cavity.) |
Discussion
Ameloblastic carcinoma is an extremely rare epithelial tumor of odontogenic origin located in the mandible or maxilla, with a predilection for the mandible [1]. There are little data on this pathology because few cases have been reported. According to a meta-analysis of 199 cases in 2017, ameloblastic carcinoma occurs primarily at the age of ∼40 years, with 2.4-fold male dominance [1]. It may be primary or secondary to pre-existing benign ameloblastoma, as described by Lin et al. [2]. Primary ameloblastic carcinoma is the most common. Clinically, it can manifest as pain, rapidly growing swelling, bleeding, ulceration, tooth mobility, trismus, labiomental paresthesia, dysphonia, or dysphagia [3]. Histological examination is pivotal to confirm diagnosis. It is a malignant epithelial tumor that retains the characteristics of ameloblastic differentiation but also shows cytological characteristics of malignancy [3]. For diagnosis, most studies use immunohistochemical analysis of Ki67—a frequently elevated marker in ameloblastic carcinoma—which is an index of tumor proliferation [4–7]. This is an additional aid for the differential diagnosis of ameloblastomas. Bello et al. [5] have examined different epithelial and stromal prognostic markers for ameloblastic carcinoma and solid multicystic ameloblastoma and concluded that Ki67 expression is significantly higher in ameloblastic carcinoma than in ameloblastoma. In the present case, histological examination was conducted outside the hospital monitoring facility and did not report assessments of any of these markers. There is no histological distinction between primary and secondary ameloblastic carcinoma. In 2005, the World Health Organization classification included ameloblastic carcinoma in ameloblastic tumors and described three subcategories: primary, secondary, and peripheral types. The new classification of odontogenic lesions in 2017 (4th edition) [8] (Fig. 6) reclassified it in the group of malignant odontogenic tumors discarding the previous subcategories, which are considered a morphological continuum because of their similar behavior. Imaging is non-specific and only has diagnostic value in locoregional extension examination. Management remains controversial, although surgery with healthy margins of exeresis offers the best prognosis and remains the treatment of choice. Most studies are in favor of surgery, followed by radiotherapy. Giridhar et al. [1] have shown that tumor resection with healthy R0 margins was associated with favorable survival without the need for adjuvant radiotherapy, except in older people who present more risk factors. The effectiveness of radiotherapy remains controversial. Jensen et al. [9] have shown complete remission of the disease with high dose of radiation (60 Gy) and concluded that it offered an alternative to surgery. According to retrospective study of radiotherapy alone for recurrence (old surgery) and adjuvant radiotherapy after surgery [10] in 2016, radiotherapy offered the potential for disease control when exeresis margins were affected or when recurrence was noted. In other studies [6–11], this approach was reportedly ineffective. In our case, radiotherapy seemed to have stabilized the course of the disease, as seen on the last scan. However, decreased hearing acuity seemed to be a complication of radiation therapy and not tumor progression given the absence of radiological evolution of the tumor. The tumor evolves via either a rapid and aggressive local extension or a distant metastatic lesion, particularly pulmonary, appearing in the 4th to 12th month after the surgery [6]. The bone, liver, and brain are other metastatic sites. The prognosis is reserved, with a high recurrence rate [7] and overall survival rates of 87.16% and 69.08% at 2 and 5 years, respectively [1]. Careful long-term follow-up is, therefore, essential in the management of this tumor [11].
Conclusion
For ameloblastic carcinoma, surgery remains the treatment of choice to date, sometimes combined with adjuvant radiotherapy, because it offers the best survival potential and prognosis. The effectiveness of radiation therapy remains controversial across various studies; however, this could be an alternative to surgery when it is not feasible and could provide good local control of the disease, as presented in this clinical case.
Conflicts of interests
The authors declare that they have no conflicts of interest in relation to this article.
References
- Giridhar P, Mallick S, Upadhyay AD, Rath GK. Pattern of care and impact of prognostic factors in the outcome of ameloblastic carcinoma: a systematic review and individual patient data analysis of 199 cases. Eur Arch Otorhinolaryngol 2017;274:3803–3810. [CrossRef] [PubMed] [Google Scholar]
- Lin Z, Chen F, Wang T, Hu Q, Sun G. The variability and complexity of ameloblastoma: carcinoma ex ameloblastoma or primary ameloblastic carcinoma. J Cranio-Maxillofac Surg 2013;41:190–193. [CrossRef] [Google Scholar]
- Yoon H-J, Hong S-P, Lee J-I, Lee S-S, Hong S-D. Ameloblastic carcinoma: an analysis of 6 cases with review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 2009;108:904–913. [CrossRef] [Google Scholar]
- Safadi RA, Quda BF, Hammad HM. Immunohistochemical expression of K6, K8, K16, K17, K19, maspin, syndecan-1 (CD138), α-SMA, and Ki-67 in ameloblastoma and ameloblastic carcinoma: diagnostic and prognostic correlations. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;121:402–411. [CrossRef] [PubMed] [Google Scholar]
- Bello IO, Alanen K, Slootweg PJ, Salo T. Alpha-smooth muscle actin within epithelial islands is predictive of ameloblastic carcinoma. Oral Oncol 2009;45:760–765. [CrossRef] [PubMed] [Google Scholar]
- Dutta M, Kundu S, Bera H, Barik S, Ghosh B. Ameloblastic carcinoma of mandible: facts and dilemmas. Tumori 2014;100:e189–e196. [CrossRef] [PubMed] [Google Scholar]
- Loyola AM, Cardoso SV, de Faria PR, Servato JPS, Eisenberg ALA, Dias FL, et al. Ameloblastic carcinoma: a Brazilian collaborative study of 17 cases. Histopathology 2016;69:687–701. [CrossRef] [PubMed] [Google Scholar]
- Soluk-tekkesin M, Wright JM. The World Health Organization classification of odontogenic lesions: a summary of the changes of the 2017 (4th) edition. Head Neck Pathol 2017;11: 68–77. [Google Scholar]
- Jensen AD, Ecker S, Ellerbrock M, Nikoghosyan A, Debus J, Münter MW. Carbon ion therapy for ameloblastic carcinoma. Radiat Oncol 2011;6:13. [CrossRef] [PubMed] [Google Scholar]
- Kennedy WR, Werning JW, Kaye FJ, Mendenhall WM. Treatment of ameloblastoma and ameloblastic carcinoma with radiotherapy. Eur Arch Otorhinolaryngol 2016;273:3293–3297. [CrossRef] [PubMed] [Google Scholar]
- Routray S, Majumdar S, Swain N. Ameloblastic carcinoma: an effort to abridge this diagnostic challenge. Indian J Cancer 2015;52:234. [CrossRef] [PubMed] [Google Scholar]
All Figures
 |
Fig. 1 Histological section of the biopsy showing cells with elongated nuclei assuming a palissadic appearance with clarified cells having an inverted nuclear polarity. The nuclei are the site of clear-cut nuclear atypia, and mitotic figures are visible. (a) Magnification ×50, (b) Magnification ×100, (c) Magnification ×200, (d) Magnification ×400. |
| In the text | |
 |
Fig. 2 Panoramic radiography showing diffuse osteolysis of the ascending branch of the left mandible 5 years after the first consultation. (Panoramic radiography showing diffuse osteolysis of the ascending branch of the left mandible 5 years after the first consultation.) |
| In the text | |
 |
Fig. 3 Scan of facial bone window and in axial section at the mandibular level, at 5 years, showing an osteolytic and expansive lesion of the ascending branch of the left mandible. (Scan of facial bone window and in axial section at the mandibular level, at 5 years, showing an osteolytic and expansive lesion of the ascending branch of the left mandible.) |
| In the text | |
 |
Fig. 4 T1 time MRI in axial section, at 6 years, through the mandible, showing the expansive process in relation to the ascending branch of the mandible. (T1 time MRI in axial section, at 6 years, through the mandible, showing the expansive process in relation to the ascending branch of the mandible.) |
| In the text | |
 |
Fig. 5 Facial scan (bone window) in axial section 1 year after radiotherapy showing a stabilization of this expansive process and communication with the oral cavity. (Facial scan (bone window) in axial section 1 year after radiotherapy showing a stabilization of this expansive process and communication with the oral cavity.) |
| In the text | |
 |
Fig. 6 Table of the new 2017 classification of odontogenic tumors [8]. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


