| Issue |
Med Buccale Chir Buccale
Volume 23, Number 3, October 2017
|
|
|---|---|---|
| Page(s) | 164 - 168 | |
| Section | Cas clinique et revue de la littérature / Up-to date review and case report | |
| DOI | https://doi.org/10.1051/mbcb/2017009 | |
| Published online | 27 October 2017 | |
Up-to Date Review And Case Report
Ameloblastic fibrosarcoma of the mandible: case report and literature review
1
Maxillofacial Surgery and Stomatology Department, CHU Hôtel Dieu,
1 place Alexis Ricordeau,
44093
Nantes, France
2
Odontology Department, CHU Hôtel Dieu,
1 place Alexis Ricordeau,
44093
Nantes, France
3
Maxillofacial Surgery and Stomatology Department, Pole Santé Sud,
28 rue de Guetteloup,
72000
Le Mans, France
* Correspondence: docteur.guiol@gmail.com
Received:
20
January
2017
Accepted:
27
April
2017
Introduction: Ameloblastic fibrosarcoma is a rare malignant odontogenic tumor. Over 50% reported cases have histological evidence of ameloblastic fibroma at the same site. The mortality rate of the tumor is 19% and its recurrence rate is 37%, which mandates a total resection along with long-term follow-up. Observation: We present the case of a 14-year-old male diagnosed with ameloblastic fibrosarcoma, who underwent multiple stages of treatments and was followed up over 8 years. The initial management consisted of a total tumor resection with chemoradiotherapy. Then, for bone, soft, mucosal, and dental restoration after resection, mandibular reconstruction with a fibula free flap, onlay iliac graft, iliac graft fixation, facial lipofilling treatments (two), vestibular deepening procedures (two), and implant-supported dental prosthesis were performed. Comments and conclusion: This is the first case reported in the literature for the global management of mandibular ameloblastic fibrosarcoma, from oncological treatment to functional and aesthetic long-term rehabilitation.
Key words: ameloblastic fibrosarcoma / facial reconstruction / dental implant
© The authors, 2017
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Ameloblastic fibrosarcoma is a rare, aggressive malignant odontogenic tumor. It is defined by the World Health Organization as a neoplasm with similar structure to ameloblastic fibroma, but in which the ectomesenchyme has the characteristics of a sarcoma. In >50% reported cases of ameloblastic fibroma, malignant transformation is observed. Ameloblastic fibrosarcoma has a mortality rate of 19% and a 37% recurrence rate, thus requiring radical initial treatment with regular follow-up.
We report the case of a young patient treated for mandibular fibrosarcoma with diagnostic and treatment approaches based on oncological treatment and functional and aesthetic rehabilitation. We compared our experience in this case with the data in the literature in terms of the overall management of this tumor.
Observation
A 14-year-old boy was referred following the appearance of a painful gingival inflammatory lesion on the left side of the mouth. Nine months earlier, he had undergone intraoral enucleation of an ameloblastic fibroma at the level of the left mandibular ramus.
Clinical examination revealed a tender, inflammatory, gingival swelling between teeth 34 and 35 (Fig. 1). Orthopantomogram showed a lacunar, multilocular radiolucency mandibular lesion on the left side, pushing against the dental roots of teeth 34 and 35 (Fig. 2A). Contrast-enhanced cervicofacial CT scan showed a hypervascular left mandibular lesion measuring 31 mm × 23 mm × 25 mm, destroying the inner mandibular cortex. There was no sign of local metastasis. Magnetic resonance imaging (MRI) showed a large lesion in the left mandibular body, involving teeth 34, 35, and 36, which followed the lingual region and the mandible depressor and masseter muscles (Fig. 2B). Submandibular lymphadenopathy was detected on the left side. A biopsy of the mandibular lesion was performed, and histopathological examination found a high-grade ameloblastic fibrosarcoma (grade 3 according to the classification of the national federation of cancer centers). Immunohistochemical study on deparaffinized sections showed a positive reaction to the anti-vimentin antibody and >50% nuclear staining for MiB1 antibody. Staining for the following antibodies was negative: anti-smooth muscle actin, anti-desmin, anti-PS-100, and anti-cytokeratin.
Considering tumor size and evolution, neoadjuvant chemotherapy consisting of ifosfamide and doxorubicin was implemented for three sessions. After a 60% reduction of the initial tumor volume, an interrupted mandibulectomy between teeth 32 and 33 as well as 37 and 38 was performed, combined with left-sided cervical lymph node dissection and implementation of a mandibular reconstruction plate. Histopathological examination confirmed the diagnosis of ameloblastic fibrosarcoma with a positive anterior resection margin. All lymph node samples were negative for the tumor. One month after the first surgery, a revision of the resection margin was carried out. At histopathological examination, the margins were tumor free. Adjuvant chemotherapy was administered following surgery. It started with two sessions of ifosfamide and doxorubicin followed by two sessions of ifosfamide alone, coupled with photon intensity-modulated radiotherapy, a conformational radiotherapy in photon intensity modulation, with a total tumor radiation dose of 50.4 Gy. Response to multimodal therapy was complete. The patient was then followed up regularly to evaluate clinical progress and planning for mandibular reconstruction.
The patient presented with facial asymmetry because of the retraction of skin and subcutaneous tissues in the region that underwent surgical treatment and radiotherapy. We decided to perform a left mandibular reconstruction by a flap micro-anastomosis using a fibular flap (Fig. 3). One and a half years later, abone reconstruction using an onlay graft, using an iliac bone graft, was performed. Two years later, the mandibular deformity persisted (Fig. 4). Two facial and cervical adipocyte grafts were then performed. Two vestibular deepening procedures were then carried out to restore the vestibular bone and prosthetic space for dental restoration. To achieve this, a resin prosthesis was attached to the mandible using a perimandibular steel wire. After the procedure, the patient had infection in the vestibular deepening site associated with an exposure of the fibular graft. Local drainage complemented by antibiotic therapy was performed. Controlled wound healing allowed for mucosal healing. Despite this, the patient could not support his partial removable prosthesis, which hardly fitted the local anatomy. It was then decided to perform a fixed implant restoration.
An implant rehabilitation by screw-retained prosthesis was considered. CBCT allowed to assess the position of future implants based on the prosthetic design. In September 2015, three tissue-level implants (Straumann, Basel, Switzerland) were implemented for the teeth 33, 35, and 36. Loading of the bridge took place 3 months after. A mesial extension was carried out because the site of tooth 32 was not conducive to the implementation of an implant of sufficient diameter (Fig. 5). Radiological follow-up after 1 year showed the absence of peri-implant resorption (Fig. 6). The patient was satisfied both functionally and aesthetically. The anatomy of the bridge allowed good hygiene around the individual abutments. The patient was followed up on an annual basis both for cancer and implant statuses. Eight years after the tumor diagnosis, the patient was in complete remission.
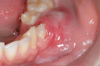 |
Fig. 1 Clinical appearance of the tumor from the gingival aspect at the time of diagnosis. |
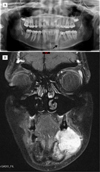 |
Fig. 2 Preoperative imaging. Orthopantomography showing multilocular radiolucency in the left mandible with the roots of teeth 34 and 35 being pushed away (A). Facial coronal magnetic resonance imaging (gadolinium-enhanced T2-weighted) showing left mandibular tumoral lesion (B). |
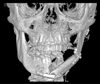 |
Fig. 3 Frontal view of a three-dimensional cone-beam computed tomography reconstruction showing fibula free flap. |
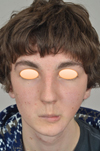 |
Fig. 4 Photograph showing facial distortion with a defect under the left mandible and lower labial retraction. |
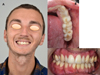 |
Fig. 5 Facial (A) and intraoral (B, C) photographs showing dental prosthetic implant restoration in sector 3. |
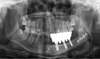 |
Fig. 6 Orthopantomography at 1-year follow-up after of implant-supported dental prosthesis implantation in sector 3. |
Comments
Ameloblastic fibrosarcoma is a locally aggressive, rare malignant odontogenic tumor [1–4]. It represents <5% of all odontogenic tumors [2,4]. In 2015, only 72 cases were reported in English literature [2]. Ameloblastic fibrosarcoma most often occurs in patients around 30 years of age [1], but reported cases include those of patients ranging from 4 months to 78 years of age. It is most common in the mandible (79% cases [4]), predominantly in its posterior part [2,5].
Ameloblastic fibrosarcoma is the malignant counterpart of ameloblastic fibroma [2–4]. New-onset cases may be diagnosed, but malignant transformation of an ameloblastic fibroma is reported in >50% cases.
It is necessary to take the history of patients, particularly the management of ameloblastic fibroma. In most cases, patients report pain [1,2,4,5], sometimes associated with paresthesias [1,4].
Ameloblastic fibrosarcoma causes intraoral and/or extraoral swelling [1,2,4,5]. On intraoral examination, the gums may be healthy, erythematous, or replaced by granulation tissue [1,2].
Radiographically, multilocular radiolucency is observed, with vague, irregular margins [1,5]. The lesion causes osteolysis with cortical perforations [5]. The preferred imaging examination is (MRI). Imaging examinations for systemic spread very rarely find metastases [1–3,5].
Histologically, this lesion is a mixed odontogenic tumor comprising a benign epithelial ameloblastic component and a malignant mesenchymal component, made up of ovoid and fusiform cells [1,5]. Hypercellularity [2–5] and nuclear polymorphisms [3,4] are observed in the mesenchymal component, associated with highly numerous images of mitoses [3,4] sometimes atypical [1]. In time, the epithelial component decreases in number and size compared to the original lesion, until it occasionally disappears [2,4]. Immunohistochemical study shows a positive marking to the anti-vimentin antibody because of fibroblastic proliferation. MiB1 antibody positivity is also usually observed, suggesting cellular proliferation. Anti-cytokeratin, anti-EMA, anti-S100 protein, and anti-desmin antibodies are all negative. To evaluate the prognosis, the National Federation of Cancer Centers considers tumor differentiation, mitotic number, and necrosis extent. It defines the grades 1–3 according to the severity of malignancy.
Initial treatment includes excision surgery combined with chemotherapy and radiotherapy. Surgery should include wide excision with negative margins [1,2,4,5]. Cervical lymph node dissection is usually not necessary [2–5] because the sarcoma extends through the vascular channels and lymphatic metastasis is absent [1,5]. In the present case, we decided to perform a lymph node dissection as the MRI had found the presence of lymphadenopathy. Neoadjuvant and/or adjuvant chemotherapy are indicated [5]. Adjuvant radiotherapy with a total dose of 50–60 Gy is used to decrease the risk of postoperative recurrence [5]. Recidivism rate for ameloblastic fibrosarcoma is 23.9–37% [1,2], hence the need for radical treatment and regular follow-up [1].
Rehabilitation is necessary the restoration of both facial symmetry and oral functions.
Oncological excisions are usually devastating. The consequences are dental losses, bone defects [6], soft-tissue deficits (loss of vestibule, floor of the mouth) [7], scarring (skin scars, intraoral scar tissue) [6], maxillomandibular relationship disorders [8], limitation of tongue movement and diction disorders [7], facial asymmetry, and motor and sensory disturbances.
Following the excision, reconstruction may be necessary to correct sequelae [2]. This may call for local flaps or in some cases free flaps. Depending on the quality of the necessary tissue, the following can be performed: fibula, scapula, or iliac crest flap, if bone contribution is required. Otherwise, all other flaps (forearm, latissimus dorsi, pectoralis major) can be used. The disadvantages of the free fibula flap are that it is nonkeratinized and the attached mucosal thickness is higher than the gum, which induces soft-tissue mobility [9]; atypical ridge shape; insufficient bone height and width (sometimes); and the need to be positioned in the prosthetic corridor.
Grafts may also be used to correct sequelae of excision. Cutaneous or mucosal grafts are often necessary to replace soft-tissue gaps. Bone grafts (cranial bone, iliac bone, mandibular bone, and tibial bone) allow for treatment of nonunion, to increase bone volumes for morphological or implant purposes. Moreover, other interventions, such as commissuroplasties, can be carried out to improve the oral opening [7], reduction of fibula bone volume [10], mucous thinning of this flap [7,10], a vestibuloplasty to recreate a prosthetic corridor and increase labial and cheek mobility [9].
Once bone and mucosal reconstruction is achieved, an implant-based rehabilitation may be performed by choosing a supra-implant prosthesis matching the patient's preference. Regarding the supra-implant prosthesis type, studies favor fixed screw-retained or cement-retained prosthetics, especially after radiotherapy, to limit mucosal support [9]. The prosthesis of choice is the multi-screw-retained prosthesis because of its many benefits. It requires less implants and allows quick disassembly in case of prosthetic problems or in the cancer management. However, it requires a sustained, sometimes delicate, hygiene because of difficulty in access and prosthetic anatomy.
Conclusion
Management of ameloblastic fibrosarcoma requires a multidisciplinary team from the surgical excision to the dentomaxillofacial rehabilitation. The dental and occlusal rehabilitation prosthetic design must be integrated from the beginning of management, and requires close collaboration between the dental and maxillofacial surgeons.
Conflicts of interests
The authors declare that they have no conflicts of interest in relation to this article.
References
- Noordhoek R, Pizer ME, Laskin DM. Ameloblastic fibrosarcoma of the mandible: treatment, long-term follow-up, and subsequent reconstruction of a case. J Oral Maxillofac Surg 2012;70:2930–2935. [CrossRef] [PubMed] [Google Scholar]
- Al Shetawi H, Alpert EH, Buchbinder D, Urken ML. Ameloblastic fibrosarcoma of the mandible: a case report and a review of literature. J Oral Maxillofac Surg 2015;73:1661.e1–1661.e7. [CrossRef] [Google Scholar]
- Muller S, Parker DC, Kapadia SB, Budnick SD, Barnes EL. Ameloblastic fibrosarcoma of the jaws: a clinicopathologic and DNA analysis of five cases and review of the literature with discussion of its relationship to ameloblastic fibroma. Med Oral Pathol Oral Radiol Endod 1995;79: 469–477. [CrossRef] [Google Scholar]
- Bregni RC, Taylor AM, Garcia AM. Ameloblastic fibrosarcoma of the mandible: report of two cases and review of the literature. J Oral Pathol Med 2001;30:316–320. [CrossRef] [PubMed] [Google Scholar]
- Chen SJ, Zheng XW, Lin X, Liu H. Ameloblastic fibro-odontosarcoma of the mandible in a pediatric patient. Eur Ann Otorhinolaryngol Head Neck Dis 2016;133:419–421. [CrossRef] [PubMed] [Google Scholar]
- Park YS, Kwon HB. Three-dimensional finite element analysis of implant-supported crown in fibula bone model. J Adv Prosthodont 2013;5:326–332. [CrossRef] [PubMed] [Google Scholar]
- Cuesta-Gil M, Ochandiano Caicoya S, Riba-García F, Duarte Ruiz B, Navarro Cuéllar C, Navarro Vila C. Oral rehabilitation with osseointegrated implants in oncologic patients. J Oral Maxillofac Surg 2009;67:2485–2496. [CrossRef] [PubMed] [Google Scholar]
- Mancha de la Plata M, Gías LN, Díez PM, Muñoz-Guerra M, González-García R, Lee GY, Castrejón S, Rodríguez-Campo FJ. Osseointegrated implant rehabilitation of irradiated oral cancer patients. J Oral Maxillofac Surg 2012;70:1052–1063. [Google Scholar]
- Bodard AG, Salino S, Bémer J, Lucas R, Breton P. Dental implant placement after mandibular reconstruction by microvascular free fibula flap: current knowledge and remaining questions. Oral Oncol 2011;47:1099–1104. [CrossRef] [PubMed] [Google Scholar]
- Marunick MT, Roumanas ED. Functional criteria for mandibular implant placement post resection and reconstruction for cancer. J Prosthet Dent 1999;82:107–113. [CrossRef] [PubMed] [Google Scholar]
All Figures
 |
Fig. 1 Clinical appearance of the tumor from the gingival aspect at the time of diagnosis. |
| In the text | |
 |
Fig. 2 Preoperative imaging. Orthopantomography showing multilocular radiolucency in the left mandible with the roots of teeth 34 and 35 being pushed away (A). Facial coronal magnetic resonance imaging (gadolinium-enhanced T2-weighted) showing left mandibular tumoral lesion (B). |
| In the text | |
 |
Fig. 3 Frontal view of a three-dimensional cone-beam computed tomography reconstruction showing fibula free flap. |
| In the text | |
 |
Fig. 4 Photograph showing facial distortion with a defect under the left mandible and lower labial retraction. |
| In the text | |
 |
Fig. 5 Facial (A) and intraoral (B, C) photographs showing dental prosthetic implant restoration in sector 3. |
| In the text | |
 |
Fig. 6 Orthopantomography at 1-year follow-up after of implant-supported dental prosthesis implantation in sector 3. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


