| Issue |
J Oral Med Oral Surg
Volume 29, Number 4, 2023
|
|
|---|---|---|
| Article Number | 43 | |
| Number of page(s) | 5 | |
| DOI | https://doi.org/10.1051/mbcb/2023042 | |
| Published online | 02 February 2024 | |
Case Report
Lingual location of Sweet's syndrome: A case report
1
Oral Pathology and Surgery Department, CHU, Oran, Algeria
2
Department of Dental Medicine / Faculty of Medicine / Oran University Oran, Algeria
3
Conservative Dentistry and Endodontics Research Laboratory Oran, Algeria
4
Pediatric Accidentology Research Laboratory Oran, Algeria
5
Oral Biology Research Laboratory Oran, Algeria
* Correspondence: selma.messirdi@yahoo.com
Received:
31
October
2022
Accepted:
24
September
2023
Context: Sweet's syndrome is a rare acute febrile neutrophilic dermatosis and idiopathic in two-thirds of cases. The pathophysiological mechanisms of Sweet's syndrome are poorly understood. Observation: A 35 yr old female patient has been referred from the Dermatology Department to the Oral and Surgery Department, University Hospital Center of Oran (Algeria), for a lingual nodule. For medical history, the patient had a Sweet's syndrome diagnosed 1 yr ago and a rheumatoid arthritis treated with corticosteroids. An excisional biopsy of the lingual nodule was performed and the anatomopathological result revealed a pyogenic granuloma associated with polymorphonuclear neutrophilic vasculitis in the context of Sweet's syndrome. Discussion and conclusion: Sweet's syndrome is characterized by a constellation of clinical symptoms, biological and histological abnormalities and is manifested by the sudden appearance of painful skin lesions in the form of asymmetric erythematous papules, nodules or plaques. In the context of Sweet's syndrome, faced with an oral with cutaneous lesions a correlation and/or manifestation of neutrophilic dermatosis must be suspected.
Key words: Sweet's syndrome / oral pathology and surgery / neutrophilic dermatosis
© The authors, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Sweet's syndrome (SS) or acute febrile neutrophilic dermatosis was first described by Robert Douglas Sweet in 1964 [1,2] as a rare inflammatory disease with predominantly cutaneous expression, belonging to the group of neutrophilic dermatoses [3]. Its incidence is more frequent in women and predominates in middle age. However, it can occur at any age [4]. SS is idiopathic in two thirds of cases [2,5]. However, it can also be associated with myeloid hemopathies, more rarely with solid tumors, certain drugs, autoimmune diseases, autoinflammatory or infectious diseases or in a paraneoplastic context [2,5,6]. The pathophysiological mechanisms of SS are probably multifactorial but remain poorly elucidated [2,6]. Association with infectious diseases, autoimmune diseases, neoplasias or drugs argues in favor of a hypersensitivity reaction involving cytokines, interferon gamma and TNF for neutrophil activation and recruitment [3]. SS may occur places that have received pressure and trauma [4], these pathogenesis remains a hypothesis described in the literature [3,4].
We reported the case of a patient with simultaneous occurrence of Sweet's syndrome associated with a rheumatoid arthritis and a lingual nodule. In previous literature, a few cases of oral lesions have been reported in patients with neutrophilic dermatosis and were mainly associated with SS [4,7]. But to our knowledge, no patient with concurrent Sweet's syndrome, oral pyogenic granuloma associated with neutrophilic polymorphonuclear vasculitis had been reported yet in literature.
Observation
A 35 yr old female patient was referred, in January 2021, to the Department of Oral Pathology and Surgery of the University Hospital Center of Oran (CHUO), Algeria, from the Department of Dermatology of the CHUO, for the therapeutic management of a lingual nodule. The past medical history reveals a cutaneous lesions characterized by edematous and erythematous plaques on the hands, neck and face associated with joint pain. Following the investigations of the attending physicians, diagnosis of SS associated with acute rheumatoid arthritis was made in January 2020. Corticosteroids were prescribed at a dose of 0.5mg/ kg which allowed an optimal regression of the cutaneous lesions. The occurrence of the lingual nodule was synchronous with the skin manifestations that led to the diagnosis of SS. This painless nodule was located in the anterior third of the dorsal surface of the tongue on the midline. It was pedunculated, soft in consistency, covered with erythematous mucosa and progressive in evolution (Fig. 1). Through the clinical data and the history of the disease, our diagnosis was oriented towards a pyogenic granuloma of the tongue, however an oral manifestation of SS cannot be ruled out. The preoperative assessment revealed an increase in neutrophils. Treatment consisted of an excisional biopsy with complete removal of the lingual nodule followed by hemostasis and realization of two separate stitches in ‘O’. The surgical specimen was preserved in formalin for anatomopathological examination, the result was in favor of a pyogenic granuloma of the tongue with neutrophil polymorphonuclear vasculitis as part of sweet's syndrome (Figs. 2 and 3). The 10-day check-up showed optimal healing.
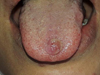 |
Fig. 1 Clinical aspect of the lingual nodule. |
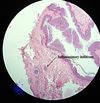 |
Fig. 2 Histological details showing the squamous epithelium and inflammatory infiltration in the chorion (Magnification ×10). Dr. Bentliba. M Anatomopathologist Doctor ( bentlibadiag@gmail.com). |
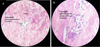 |
Fig. 3 a, b: Histological details revealing a neutrophilic polymorphonuclear vasculitis in the context of Sweet's syndrome (a: Magnification × 40, b: Magnification × 100). Dr. Bentliba. M Anatomopathologist Doctor (bentlibadiag@gmail.com). |
Discussion
SS is characterized by a constellation of clinical symptoms, biological and histological abnormalities that may be associated with various diseases [4,6]. The typical manifestations of this syndrome are high fever and the sudden appearance of painful skin lesions in the form of papules, nodules or asymmetrical erythematous plaques located preferentially on the upper extremities, of the face and neck [6]. Involvement of the oral mucosa is rare and less painful than on the skin [7]. Oral manifestations are superficial aphthoid lesions (oral mucosa and tongue), bullae and hemorrhagic vesicles (labial and gingival mucosa), gingival hyperplasia, ulcerative periodontitis, necrotic nodules (labial mucosa), papules (macerated: palate and tongue), pustules (individual and grouped: palate and pharynx), swelling (tongue), ulcers (palate) [8]. Biological abnormalities include an accelerated sedimentation rate (ESR) higher than 20 mm in the first hour, elevation of C-reactive protein (CRP), and neutrophilic hyperleukocytosis [4]. Histology reveals a diffuse infiltrate of mature neutrophils typically located in the superficial dermis [3]. The presence of a vasculitis is no longer a criterion for eliminating the diagnosis, fibrinoid necrosis of the vessel wall and leukocytoclastic can be observed in forms typical of SS. [2]. A skin and/or mucosal biopsy for routine histopathological evaluation is essential to confirm a clinically suspected diagnosis of SS [4,7]. The positive diagnosis is based on clinical, histological and biological arguments. Two major and 2 minor criteria are required for diagnosis according to the classification of Su and Liu, modified by Von den Driesch (Tab. I) [9–11]. The treatment of SS is based on anti-inflammatory drugs that allow a rapid regression of symptoms and mucocutaneous lesions [3]. Other treatments described as effective can be administered depending on the clinical situation, such as colchicine, dapsone, ciclosporin, anti-interleukin-1 and anti-tumor necrosis factor [6]. SS can be associated with other diseases such as solid tumors or pleomorphic adenoma. In this case surgical intervention is needed, no recurrence of SS lesions is reported after a complete excision of tumor [12]. The prognosis of SS varies depending on the underlying cause because this condition can be associated with other diseases, including malignancy. Generally, with timely diagnosis and appropriate treatment, the lesions of SS resolve without scarring [13]. In our case, according to the clinical and histopathological data, the diagnosis of pyogenic granuloma with a neutrophilic polymorphonuclear vasculitis in the context of SS was retained.
Pyogenic granuloma is defined as a common, acquired, benign vascular tumor according to International Society for the Study of Vascular Anomalies (ISSVA) classification 2018 [14–16]. Pyogenic granuloma is a reactive tumorlike lesion that arises in tissues such as the skin and mucous membranes (mostly oral cavity) [16]. Clinically, pyogenic granuloma presents as sessile or pedunculated exophytic mass with a smooth or lobulated surface which has a tendency to bleed easily [17]. Various factors (chronic local irritation, trauma, and hormonal changes) are implicated in the etiopathogenesis of this entity, but the exact cause is unknown [15]. Histologically, these lesions show an excessive proliferation of vascular type of connective tissue to a nonspecific infection [17]. The differential diagnosis of oral pyogenic granuloma includes other vascular tumors (hemangioma, lymphangioma) peripheral giant cell granuloma, other malignancies that can mimic pyogenic granuloma (malignant lymphomas, basal cell carcinoma, Kaposi's sarcoma, angiosarcoma, non‐Hodgkin's lymphoma) [14,15].
In our case, given the clinical criteria of the lingual nodule, we evoked as a differential diagnosis peripheral giant cell granuloma, a benign tumorlike and benign conjunctival tumors (vascular) of the oral mucosa in an isolated context not related to SS. Nevertheless, new discoveries have shed light on the role of inflammatory signaling, disease induction, and relationship with malignancy [18], that's why we keep in mind anatomopathological surprises. Although the appearance of the nodule coincides with the cutaneous manifestations of SS, however, only the histological criteria of SS allow it to be isolated or to be associated with this syndrome. The anatomopathological result of our patient revealing a neutrophilic polymorphonuclear vasculitis associated to pyogenic granuloma classified it as part of SS.
Like our case, SS has been reported to occur in the setting of a variety of autoimmune conditions among which rheumatoid arthritis [18,19]. There are several case reports indicating an association between SS and rheumatoid arthritis. Proinflammatory cytokines are considered to play a vital role in the pathogenesis of both rheumatoid arthritis and SS [19]. It is possible that co-existence of autoimmune conditions and SS is merely coincidental, and that there is no etiologic link between autoimmune disease and SS. However, as may be the case in SS occurring in the setting of infections, a dysregulated immune system in autoimmune states lends itself well to the development of SS and maybe reported to trigger of this syndrome [18]. Moreover, it is possible that the concomitantly occurrence of the nodule in the setting of SS be merely a coincidence. However, in addition, of the fact that SS and pyogenic granuloma share the same trauma pathogenic hypothesis (trauma). mechanistically, it is possible and plausible that SS sets up a pro-inflammatory milieu, that is propitious to the development of this pyogenic granuloma associated with neutrophilic polymorphonuclear vasculitis.
In the literature and in the context of oral SS, Cohen indicates in his article published in 2003, nodular necrotic lesions especially in the labial mucosa as well as aphthoid and ulcerative lesions especially in the oral mucosa and the tongue. Popular lesions and swelling have been described especially in the tongue area [4]. Kato and al., in 2008, found oral erosions [20], while Fricain and al., in 2015, observed recurrent oral ulcers associated with lingual pustulosis [7]. Bangaru and al., in 2022, reported one case associated with pleomorphic adenoma [12]. In our case, clinically, we found a lingual nodule who's the histopathological reports associated a pyogenic granuloma with a neutrophilic polymorphonuclear vasculitis correlated to SS (Tab. II). Like Bangaru and al., in 2022, No recurrence of SS lesions of our case is reported after drug therapy based on corticosteroid therapy and a complete excision of tongue tumor.
Conclusion
SS has been reported in conjunction with a host of autoimmune and pro inflammatory states; it is unclear, however, whether these associations are due to a shared etiology or are just coincidental occurrences [17].
Although rare, the inaugural symptoms of SS can be found in extracutaneous sites, including the oral mucosa. The diagnosis of SS is based on the presence of clinical, biological, and histological abnormalities showing sterile neutrophilic inflammation in the involved organ and sometimes vasculitis that are no longer a criterion for eliminating the diagnosis can be revealed by anatomopathological exam.
In the context of diagnosed SS, any oral lesion should be correlated with this syndrome until proven otherwise. Faced with an oral lesion with cutaneous lesions, correlation and/or manifestation of neutrophilic dermatosis must be suspected.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This research did not receive any specific funding.
Ethical approval
Ethical approval was not required.
Informed consent
The authors declare that informed consent was not required.
Authors contributions
All authors contributed equally in the article.
References
- SweetRD. An acute febrile neutrophilic dermatosis. Br J Dermatol 1964;76:349–356. [CrossRef] [PubMed] [Google Scholar]
- ChellyI, ZehaniA, Mbazaa A, et al. Vascularite au cours du syndrome de Sweet. La Tunisie Médicale 2012;90,08:636–640. [Google Scholar]
- Bouzidi H, Gallouj S, Amraoui N, Mernissi FZ, Harmouch T. Syndrome de Sweet: étude clinique et anatomopathologique sur 5 ans. Pan Afr Med J 2015;20:362. [PubMed] [Google Scholar]
- Cohen PR. Sweet's syndrome: a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J. Rare Dis 2007;2,34: 1–28. [CrossRef] [Google Scholar]
- Ahangari D, Ngendahayo P, Colinet B, Roquet-Gravy PP. Syndrome de Sweet chez une patiente traitée par erlotinib pour un adénocarcinome bronchique. Ann Dermatol Venereol 2020;147: 202–106. [CrossRef] [PubMed] [Google Scholar]
- Lang C, Quenan S. Syndrome de Sweet : un diagnostic à ne pas manquer. Rev Med Suisse 2017;13:678–683. [PubMed] [Google Scholar]
- Fricain JC, Sibaud V, Lepreux S, Taieb A, Boralevi F, D'Elbée JM. Sweet's syndrome revealed by oral pustulosis. Med Buccale Chir Buccale 2015;21:177–218. [CrossRef] [EDP Sciences] [Google Scholar]
- Cohen PR, Kurzrock R. Sweet's syndrome revisited: a review of disease concepts. Int J Dermatol 2003;42:761–778. [CrossRef] [PubMed] [Google Scholar]
- Von den Driesch P. Syndrome de Sweet (dermatose aiguë fébrile à neutrophiles). J Ann Acad Dermatol 1994;31:535–556. [CrossRef] [Google Scholar]
- Rjeibi I, Zeglaoui F, El fekih N, Ezzine N, Fazaa B, Kamoun MR. Le syndrome de sweet : à propos de 5 observations. Rev Med Liege 2006;61,12:834–836. [PubMed] [Google Scholar]
- Chelly I, Zehani A, Mbazaa A, Azouz H, Nfoussi H, Kchir N, et al. Syndrome de Sweet : étude rétrospective de 47 cas. Rev Med Int 2013;34,4:197–201. [CrossRef] [Google Scholar]
- Bangaru H, Raghavendra KR, Shankar S. Sweet's syndrome: a study of 16 cases and review of literature. Clin Dermatol Rev 2022;6:121–126. [CrossRef] [Google Scholar]
- Vashisht P, Goyal A, Hearth Holmes MP. Sweet Syndrome. Treasure Island (FL): StatPearls; 2023. [Google Scholar]
- Wollina U, Langner D, França K, Gianfaldoni S, Lotti T, Tchernev G. Pyogenic granuloma: a common benign vascular tumor with variable clinical presentation: new findings and treatment. Maced J Med Sci 2017;25:423–426. [CrossRef] [Google Scholar]
- Sarwal P, Lapumnuaypol K. Pyogenic Granuloma. Treasure Island (FL): StatPearls; 2022. [Google Scholar]
- Matariya R, Parmar S, Chauhan NA, Irfan M, Rao N. Oral Pyogenic granuloma-a case report and review of literature. Bull Env Pharmacol Life Sci Spl 2022;1:201–204. [Google Scholar]
- Parikh K, Shah P, Shah M. Pyogenic granuloma: a report of three cases. J Dent Sci 2011;2:53–55. [Google Scholar]
- Joshi TP, Friske SK, Hsiou DA, Duvic M. New practical aspects of sweet syndrome. Am J Clin Dermatol 2022;23:301–318. [CrossRef] [PubMed] [Google Scholar]
- Paul P, Walker CP, Paul M, Dey D. Sweet's syndrome in a patient with seropositive rheumatoid arthritis after starting adalimumab: is sweet's syndrome related to rheumatoid arthritis or is it the paradoxical effect of adalimumab? Cureus 2021;13:8. [Google Scholar]
- Kato T, Kawana S, Takezaki SI, Kikuchi S, Futagami A. Case of Sweet's syndrome with extensive necrosis and ulcers accompanied by myelodysplastic syndrome. J Nippon M-ed Sch 2008;75,3:162–165. [CrossRef] [PubMed] [Google Scholar]
All Tables
All Figures
 |
Fig. 1 Clinical aspect of the lingual nodule. |
| In the text | |
 |
Fig. 2 Histological details showing the squamous epithelium and inflammatory infiltration in the chorion (Magnification ×10). Dr. Bentliba. M Anatomopathologist Doctor ( bentlibadiag@gmail.com). |
| In the text | |
 |
Fig. 3 a, b: Histological details revealing a neutrophilic polymorphonuclear vasculitis in the context of Sweet's syndrome (a: Magnification × 40, b: Magnification × 100). Dr. Bentliba. M Anatomopathologist Doctor (bentlibadiag@gmail.com). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


