| Issue |
J Oral Med Oral Surg
Volume 29, Number 4, 2023
|
|
|---|---|---|
| Article Number | 40 | |
| Number of page(s) | 5 | |
| DOI | https://doi.org/10.1051/mbcb/2023037 | |
| Published online | 23 January 2024 | |
Case Report
Oral lichen planus pemphigoid : a case report of a rare disease treated by topical Tacrolimus mouthwash and Hydroxychloroquine
Hôpital Pellegrin, Bordeaux, France
* Correspondence: jeremydouley@gmail.com
Received:
26
October
2023
Accepted:
6
November
2023
Introduction: Oral Lichen Planus Pemphigoid (LPP) is the rare association of lichen planus and Mucous Membrane Pemphigoid (MMP) whose therapeutic lines are poorly defined. Bilateral and symmetrical reticulated white lesions characteristic of lichen planus (LP) most often precede the painful post-bullous ulcerations of pemphigoid (P). The oral LPP is very rare and the treatment is not codified. This report described a rare case of oral LPP, successfully treated with an original combo of topical Tacrolimus and hydroxychloroquine. Observation: A 72 yr old patient was referred to the unit of the oral mucosa pathology and oro-facial pain, of the oral surgery service of Bordeaux hospital. He has painful mouth ulcerations accompanied by reticulated and symmetrical white lesions. A biopsy to perform an anatomopathological examination and a direct immunofluorescence founds the anatomopathological characteristics of LP and P.As first line of treatment, local and general corticosteroid therapy was undertaken to reduce inflammation. The result was not totally satisfactory. As second line of treatment topical Tacrolimus and hydroxychloroquine made it possible to reduce drastically the patient's erosive and ulcerated lesions. Conclusion: The combination of topical tacrolimus and HCQ should be considerated as second line of treatment for LPP resistant to corticotherapy.
Key words: oral lichen planus pemphigoid / tacrolimus / hydroxychloroquine / mucous membrane memphigoid
© The authors, 2023
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Oral lichen planus (OLP) is a chronic inflammatory disease characterized clinically by bilateral and more or less symmetrical lesions, gray/white reticulated networks and histologically by a banded inflammatory infiltrate limited to the superficial part of the chorion, consisting mainly of lymphocytes [1].
Mucous membrane pemphigoid (MMP) or cicatricial pemphigoid is a rare autoimmune subepidermal blistering disorder characterized by a predominant involvement of the external mucosal surfaces, with different clinical variations: predominant ocular disease, predominant oral involvement (oral pemphigoid), as well as generalized mucocutaneous involvement. In the mouse, it's clinically resulting in post-bullous ulcerations.
The Lichen Planus Pemphoid (LPP) is an uncommon acquired autoimmune disease with features of both LP and MMP for which epidemiological data are lacking. The lesions are mainly cutaneous, more rarely mucous: oral, anal also vulvar [2].
A literature review [3] involving 78 cases of LPP reports 28 cases of skin lesions associated with oral lesions, including 4 in children, and another review [4] involving 27 cases of LPP describes only one case with oral lesions without lesions associated skin.
Clinically, cutaneous LPP is characterized by the appearance of blisters on lichenoid lesions and on uninvolved skin with a more acral distribution of bullous lesions. The main symptoms of oral LPP are white streaks, spots, bubbles and erosive lesions [3].
If first-line treatment with local corticosteroid therapy for symptomatic lesions of OLP is well codified [1], that of severe or resistant lesions is less so: topical tacrolimus, general corticosteroid therapy, immunosuppressants like methotrexate, hydroxychloroquine…
Concerning oral MMP in France, the Protocole National de Diagnostic et de Soins (PDNS) [15] recommends the combination of local treatment with topical corticosteroid therapy combined with background treatment with dapsone or sulfasalazine in second intention. We can also cite immunosuppressants such as cyclophosphamide, used in forms of MMP with advanced ocular damage or even mycophenolate mofetil, preferred for multimucosal or dapsone-resistant forms.
The effectiveness of tacrolimus and HCQ in the treatment of OLP is proven [5,6] but their use in the context of LPP is rarer and has not been described for HCQ in oral LPP.
We report a rare case of oral LPP for which we propose a new therapeutic approach with the combination of topical tacrolimus and systemic hydroxychloroquine, two therapies that have proven effective in the treatment of severe or resistant OLP.
Observation
A 72 yr old patient was referred by his dental surgeon for painful oral lichen resistant to topical corticosteroids. He didn't have any significant family antecedent. He had a notable history an anal lichen followed by his dermatologist, treated with topical corticosteroid (Clobetazol). His dental surgeon suggested oral lichen, given the history of anal lichen. He prescribed a course of bethametazone tablets but this treatment proved ineffective. Given the lack of response to first-line treatment and the patient's pain, he referred him to the oral mucosa pathology consultation at the oral surgery department of Bordeaux University Hospital.
At the first consultation in the oral mucosal pathology unit, the patient presented oral bilateral, symmetrical and reticulated white lesions accompanied by ulcerations of the internal surfaces of the cheeks, the oropharynx and the bottoms of the maxillary and mandibular vestibules (Fig. 1a). Faced with this presumptive diagnosis of active bullous lichen, a mouthwash solution of prednisolone (Solupred) was then prescribed as well as an oral treatment of prednisone (Cortancyl) at 60 mg/day with a gradual decrease over 3 months.
At the second consultation three months later, the patient described an improvement in the symptoms but the ulcerations persisted (Fig. 1b). The ulceration of the lower lip had a post-bullous appearance. Due to the poor response to treatment and the post-bullous appearance of the ulcerations, a biopsy of the lower lip at distance from ulceration was performed for conventional histological examination and direct immunofluorescence. The pathological examination of HES-stained sections revealed a lichenoid submucosal mononuclear infiltrate and supra-basal detachment. Direct immunofluorescence examination revealed continuous deposits of IgG and C3 along the basement membrane. A diagnostic of oral LPP was made and systematically, the patient was refered to an ophthalmologist to perform an ocular examination to eliminate ocular pemphigoid. The treatment was changed by a topic mouthwash of tacrolimus (Modigraf 1 mg in 100 mL of water) three times/day and oral systemic hydroxychloroquine (Plaquenil) 400 mg/day for three months (after ocular and cardiologic advice). A dose of 5 mg of prednisone was maintained.
At the third consultation, clinical improvement of the lesions thanks to the new treatment was observed (Fig. 1c): reduction of the cheek's ulcerations but persistent ulcers at the mandibular level linked to a temporary removable prosthesis made following teeth extraction by his dentist.
At the fourth consultation, the patient described an amelioration of more than 95% of the symptoms. He didn't have any pain a can eat spicy or acidic food. He had the consultation with ophthalmologist that didn't find any ocular lesion linked to pemphigoid. Due to the improvement of the symptoms, the prednisone was stopped and relayed by hydrocortisone before the synacthene test that will allow to definitively stop oral steroids.
At the fifth consultation 10 months after the patient's first visit (Fig. 1d), the size of erosive and ulcerated lesions has significantly decreased.
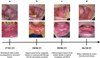 |
Fig. 1 Evolution of oral LLP lesions under treatment with topical tacrolimus and oral HCQ. |
Discussion
In this clinical case, we describe a rare case of oral mucosal LPP without associated skin lesion for which we propose a new therapeutic approach by the combination of topical tacrolimus and hydroxychloroquine (HCQ) orally, which helped to relieve the patient and considerably reduce oral lesions. Tacrolimus has previously been rarely used to treat oral LPP [7], but, to our knowledge, this is the first case of oral LPP treated with hydroxychloroquine.
These two molecules made it possible to reduce severe erosive and ulcerated lesions, unlike local and general corticosteroid therapy which is the usual treatment.
However, it is difficult to conclude on the effectiveness of the treatment in a single clinical case followed over a period of 9 months. No adverse effects were reported in this patient but longer follow-up is necessary to ensure this. In addition, it is essential to analyze the potential recurrence of the disease when treatment is reduced or even stopped.
Finally, the concomitant use of tacrolimus and HCQ does not make it possible to establish the therapeutic effectiveness of each of the molecules. This combo has been prescribed because of the severity of the case but It would be interesting to evaluate, through comparative studies, the therapeutic effect of tacrolimus alone and HCQ alone on oral LPP.
The literature data concerning LPP mainly relate to skin lesions, oral lesions being rarer.
A review of the literature on 78 cases of cutaneous LPP concerns 65 adults including 36 women and 13 children, with a mean age of 54 yr. 49 cases were successfully treated with systemic corticosteroids. Other therapies, such as dapsone (8 cases), azathioprine (10 cases), rarely erythromycin and nicotinamide, retinoids, phototherapy, erythromycin, griseofulvin or hydroxychloroquine have been used in addition to topical or systemic corticosteroids [3].
Regarding the treatments of cutaneous LPP, a literature review [8] selected 43 articles describing a total of 53 patients with cutaneous LPP. 42 patients were treated with systemic corticosteroid therapy (prednisolone) at varying doses (up to 2 mg/kg/day) and 38 of them responded effectively to the treatment. 20 other patients were treated with topical corticosteroid therapy, 15 of them successfully. Also, dapsone was used on 15 patients, 13 of which were effective. Immunosuppressants are less common, with 3 patients treated with cyclosporin and mycophenolatmofetil. Finally, 1 patient was treated with hydroxychloroquine without success and 1 other with topical tacrolimus with success. The use of these two molecules in the treatment of LPP therefore remains very sporadic.
Concerning oral LPP, a review of the literature [4] covering 27 cases of LPP with oral lesions reported a median age of 47 yr, with a female predominance (19 out of 27). Only 1 case reported oral lesions without associated skin lesions. It describes several cases of oral LPP, all treated first-line with local corticosteroid therapy (dexamethasone mouthwash or clobetasol gel). A 49 yr old man with type 2 diabete is relieved by this treatment but will require increased monitoring of his blood sugar following hyperglycemia. In a 61 yr old woman, second-line use of topical tacrolimus 0.1% resulted in clinical improvement of the lesions (disappearance of the erythema). Finally, a 50 yr old man whose response to clobetasol was insufficient was treated with dapsone, with good effectiveness, then with mycophenolate mofetil because he suffered from fatigue and nausea.
Tacrolimus belongs to the macrolide family. Its immunosuppressive action is similar to cyclosporine but it penetrates deeper into the mucosa and is 10 to 100 times more powerful than it. It inhibits the first step of T cell activation by binding to the immunophil FKBPB 12, forming a calcineurin phosphatase inhibitor complex, and thus reduces the immune response.
Its topical immunosuppressive action has a beneficial effect on the clinical improvement of erythematous, erosive or ulcerated lesions resistant to topical corticosteroids of the LP, MMP or LPP. It can be used it in the form of a 0.1% concentrated gel 2 times daily [5] or, like in this case, as a mouthwash (1 mg granules in 100 mL of water) two or three times a day depending on the initial severity of the lesions.
The first line of treatment for OLP is injected or topical corticosteroid therapy, but each patient's response varies. On lesions resistant to this conventional therapy, such as those described in this clinical case, the effectiveness of topical tacrolimus 0.1% applied twice daily allowed the reduction of ulcerated lesions in 94% of 50 patients over a mean duration period of 19.8 months [6]. The side effects were burning on the site upon application (16%), a change in taste (8%) and reverse pigmentation of the treaty area (due to an increase in the melanocytes number and the melanogenesis) [5,9]. No significant, long-standing changes in hepatic or renal biochemistry were observed [6]. The phenomenon of burning when applying tacrolimus cream can be reduced by using it as a mouthwash.
There have been only a few reports on the use of tacrolimus in MMP, with only one previous case with localized oral involvement. An 84 yr old woman with extensive oral MMP lesions performs 2 tacrolimus mouthwashes for 5 min twice daily and returns 2 months later with complete resolution of the ulcerations. The quiescent appearance of the lesions over the following months made it possible to reduce the number of mouthwashes to 1 every 2 days [10].
Another article reports the case of a 67 yr old man with MMP lesions resistant to topical and systemic corticosteroid therapy, which was stopped quickly following the appearance of osteoporosis. After trying retinoids and chloroquine without success, the use of tacrolimus 0.1% ointment for 15 min twice a day leads to the disappearance of the lesions in 3 months [11].
Regarding the use of tacrolimus to treat oral LPP, a literature review of 16 cases of oral LPP cites 9 cases treated with tacrolimus alone or combined with local corticosteroid therapy or dapsone. 12 patients benefited from partial or complete remission of the lesions [7].
Hydroxychloroquine (Plaquenil) is a slow-acting antirheumatic drug with analgesic and anti-inflammatory properties, particularly used in the treatment of rheumatoid arthritis and lupus. Hydroxychloroquine blocks T lymphocyte responses to mitogen-induced stimulation and inhibits the production of certain cytokines, interferon α and tumor necrosis factor (TNFα).
This molecule is used for its immunosuppressive activity in pathologies such as OLP at an initial dosage of 200 mg per day but can be increased to 400 mg if there is no improvement [6].
In 1993, Eisen et al. [6] were the first to use Plaquenil to treat OLP through an open trial. For 6 months, 10 patients took 200 mg of HXQ daily, increasing at 2 months to 400 mg per day if there was no improvement. The results were conclusive since 9 of the 10 patients benefited from clinical improvement, and 3 of the 6 patients with erosive OLP were cured. The addition after 6 months of topical corticosteroid therapy allowed the disappearance of persistent erosions.
A very promising more recent double-blind controlled clinical study [12] developed an HCQ gel formulation and compared a group of 11 patients with OLP who applied the gel once a day for 4 months to a 5 patients group with placebo. The treated group benefited from a 64.28% reduction in the size of lesions and the average score of pain was reduced from “4” to “1”, suggesting that these niosomal formulations could constitute a promising approach for the topical treatment of oral lichen planus in short time with less side effects and no recurrence after stopping the treatment.
The therapeutic effectiveness of HCQ is confirmed but it is necessary to carry out longer-term research to better anticipate potential recurrences and adverse effects.
A retrospective review [13] of 100 cases of OLP treated with HCQ reports 17 mild and transient side effects in 16 patients: three abdominal discomfort, three blurred vision, two rash, two nausea, two headache, two pruritis, one hair-thinning, one dizziness and one mood change. A study also reports a case of skin hyperpigmentation [14].
To our knowledge, there are no cases of MMP treated with HCQ.
Like OLP, oral MMP can be treated locally with local corticosteroid therapy, but certain severe forms of MMP (ocular, laryngeal involvement) or resistant require systemic medication.
The common treatment used to treat severe or resistant MMP lesions is dapsone, a sulfone antibiotic. However, in addition to the nausea and fatigue that it can cause [4], it is a very restrictive treatment because, to prevent the risk of hemolytic anemia and methemoglobinemia, it requires strict monitoring: a blood count with measurement of methemoglobinemia. is carried out every week for the first month then once a month for 6 months. Other treatments such as sulfasalazine, systemic corticosteroid therapy, immunosuppressants (cyclophosmamide, mycophenolate mofetil) or perhaps now HCQ are possible [15].
Conclusion
Oral LPP is a rare ambivalent disease with unclear therapeutic lines. We treated this pathology as a severe OLP with a combo of HCQ and Tacrolimus mouthwash. These treatments never described for oral LLP treatment should be considerated as second line of treatment after topical/systemic corticosteroid therapy.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This research did not receive any specific funding.
Ethical approval
Ethical approval was not required for this study.
Informed consent
Written informed consent was obtained from the patient.
Authors contributions
Jeremy Douley & Jean-Christophe Fricain: Writing original draft.
References
- Campana F, Lan R, Girard C, Rochefort J, Le Pelletier F, Leroux-Villet C, et al. French guidelines for the management of oral lichen planus (excluding pharmacological therapy). Ann Dermatol Vénéréologie. mars 2022;149:14–27. [CrossRef] [Google Scholar]
- Loyal J, Rashtak S. Vulvar lichen planus pemphigoides. Int J Womens Dermatol. déc 2017;3:225–227. [CrossRef] [Google Scholar]
- Zaraa I, Mahfoudh A, Sellami MK, Chelly I, El Euch D, Zitouna M, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. avr 2013;52:406–412. [CrossRef] [PubMed] [Google Scholar]
- Sultan A, Stojanov IJ, Lerman MA, Kabani S, Haber J, Freedman J, et al. Oral lichen planus pemphigoides: a series of four cases. Oral Surg Oral Med Oral Pathol Oral Radiol. juill 2015;120:58–68. [CrossRef] [Google Scholar]
- Hodgson TA, Sahni N, Kaliakatsou F, Buchanan JAG, Porter SR.Long-term efficacy and safety of topical tacrolimus in the management of ulcerative/erosive oral lichen planus. Eur J Dermatol EJD. 2003;13:466–470. [Google Scholar]
- Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. avr 1993;28:609–612. [CrossRef] [Google Scholar]
- Benzaquen M, Suter VGA, Gschwend M, Feldmeyer L, Borradori L. Mucous membrane pemphigoid of the oral lichen type: a retrospective analysis of 16 cases. J Eur Acad Dermatol Venereol [Internet. mai 2019 [cité 12 oct 2023];33(5). Disponible sur: https://onlinelibrary.wiley.com/doi/10.1111/jdv.15473 [Google Scholar]
- Hübner F, Langan EA, Recke A. Lichen planus pemphigoides: from lichenoid inflammation to autoantibody-mediated blistering. Front Immunol. 2019;10:1389. [CrossRef] [Google Scholar]
- Fricain JC, Sibaud V, Campana F, Lepreux S, Taïeb A. Mucosal pigmentation after oral lichen planus treatment with topical tacrolimus. Dermatol Basel Switz. 2005;210:229–232. [CrossRef] [PubMed] [Google Scholar]
- Lee HY, Blazek C, Beltraminelli H, Borradori L. Oral mucous membrane pemphigoid: complete response to topical tacrolimus. Acta Derm Venereol. sept 2011;91:604–605. [CrossRef] [PubMed] [Google Scholar]
- Assmann T, Becker J, Ruzicka T, Megahed M. Topical tacrolimus for oral cicatricial pemphigoid. Clin Exp Dermatol. nov 2004;29:674–676. [CrossRef] [PubMed] [Google Scholar]
- Bendas ER, Abdullah H, El-Komy MHM, Kassem MAA. Hydroxychloroquine niosomes: a new trend in topical management of oral lichen planus. Int J Pharm. 31 déc 2013;458:287–295. [CrossRef] [Google Scholar]
- Platais C, Lalagianni N, Momen S, Ormond M, McParland H, Setterfield J. Efficacy of hydroxychloroquine in oral lichen planus: a retrospective review. Br J Dermatol. 30 mars 2023;188:557–558. [CrossRef] [PubMed] [Google Scholar]
- Bhanja DB, Sil A, Chandra A, Biswas SK. Addisonian-like acrofacial hyperpigmentation following long-term hydroxychloroquine therapy in oral lichen planus. BMJ Case Rep. 28 janv 2021;14:e240727. [CrossRef] [Google Scholar]
- Pemphigoïde cicatricielle (PC), Texte du Protocole National de Diagnostic et de Soins (PNDS), Centres de référence des maladies bulleuses auto- immunes, Avril 2016. [Google Scholar]
All Figures
 |
Fig. 1 Evolution of oral LLP lesions under treatment with topical tacrolimus and oral HCQ. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


