| Issue |
J Oral Med Oral Surg
Volume 28, Number 1, 2022
|
|
|---|---|---|
| Article Number | 8 | |
| Number of page(s) | 9 | |
| DOI | https://doi.org/10.1051/mbcb/2021052 | |
| Published online | 21 February 2022 | |
Systematic Review
Efficacy of spirulina in management of oral submucous fibrosis − a systematic review
1
Department of Oral Medicine and Radiology, Government Dental College and Hospital, Nagpur 440003, India
2
Department of Pedodontics and Preventive Dentistry, Government Dental College and Hospital, Nagpur 440003, India
* Correspondence: rashmivcoolkarni@gmail.com
Received:
1
August
2021
Accepted:
27
September
2021
Objective: The aim of this systematic review was to evaluate the efficacy of spirulina in the management of oral submucous fibrosis. Methodology: Databases (MEDLINE via PubMed, Cochrane, EBSCO-host, Scopus, Science Direct, Clinical Trial Registry- India (CTRI) and Google scholar), review articles, bibliographies and related journal were searched from 1st January 2010 to 30th May 2020, using various combinations of MeSH terms and keywords. Results: A total of 5 clinical trials were analysed for the review, of which 4 were randomized controlled trials and 1 was non-randomized controlled trial. Mouth opening and burning sensation were analysed as primary outcome in all 5 studies. For both outcomes some studies reported statistically significant difference whereas others showed non-significant results on comparing with different interventions. Also, high risk of bias was observed among studies after performing quality analysis. Conclusion: Although the studies suggest efficacy of spirulina in management of OSF, but due to the high risk of bias there is a weak evidence regarding the effectiveness of spirulina in treating OSF. So, more uniform and standard trials on larger population should be carried out.
Key words: Oral submucous fibrosis / spirulina / systematic review
© The authors, 2022
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Oral submucous fibrosis (OSF) is a chronic, progressive, potentially malignant condition of the oral cavity that predominantly affects people of South-East Asian origin [1] and is characterized by a juxta-epithelial inflammatory reaction followed by fibroelastic change in the lamina propria and epithelial atrophy which leads to stiffness of the oral mucosa, trismus and inability to eat [2]. This entity has a long history of nomenclature starting from “atrophica idiopathica (tropica) mucosae oris” as given by Schwartz (1952) to the recent categorization as “Oral Potentially Malignant Disorder (OPMD) by Warnakulasuriya et al. [3,4]. But it is most commonly recognised by the term “Oral Submucous Fibrosis(OSF) coined by Joshi.
The pathogenesis of the disease is considered to be multifactorial. Various studies have confirmed areca nut as the major (and the only) risk factor of oral submucous fibrosis, among people who probably have a genetic predisposition to the disease. Areca nut is composed of alkaloids, flavonoids and trace elements which directly affect collagen metabolism. Other risk factors include chewing of smokeless tobacco, high intake of chilies, toxic levels of copper in food, vitamin deficiencies, malnutrition resulting in low levels of serum proteins and anaemia [5].
Data elucidate that, cases have increased dramatically from an estimated 2.5 million in 1980 to 14 million cases in 2010.[1] The prevalence rate of OSF range from 0.1 to 30%, varying by geographical location [6]. The prevalence of OSMF in India has been estimated to range from 0.2 to 2.3% in males and 1.2–4.6% in females, with a broad age range from 11 to 60 years [7].
Ever since its recognition as a possible precancerous lesion by Paymaster, its prevalence is increasing steadily with malignant transformation rate ranging from 7% to 13% [8]. A recent study from India has reported that 25.77% OSF cases converted to oral squamous cell carcinoma (OSCC) which is an alarming condition [9]. Teh et al. [10] gave a first and foremost evidence that genomic instability present in OSF patients correlate with OSF grade and plays a crucial role in OSF progression and conversion to malignancy. Mechanical trauma, excessive alcohol intake, smoking, presence of immune disorders, aging and association with other OPMDs (leukoplakia, oral lichen planus) are other risk factors for malignant transformation.
For such a chronic potentially malignant disorder various treatment protocols have been aimed with symptomatic approach to prevent further progression. Thus, treatment starts with educating patient regarding the ill-effects of areca nut chewing, followed by complete cessation of the habit of betel nut or tobacco chewing or intake of chillies. A wide range of treatment modalities are available, ranging from Medicinal, nutritional supplements, physiotherapy, LASERs, ultrasound, herbal medicines to surgical managements.
In spite of so many years of research, the pathogenesis of the disease is still not fully understood. Recent systematic reviews explicate that no single regime has proved to be entirely satisfactory [11,12]. So newer treatment options are continued to be tried. Spirulina is a filamentous, blue-green microalga used in daily diet of African and American natives. It contains high amounts of proteins, amino acids, essential oils, minerals, vitamins predominantly the B complex, tocopherols, carotenoids, and other flavonoids and cyanins. Its therapeutic role in health and disease have been evaluated in many conditions (diabetes, hypertension, arthritis etc.) with beneficial results [13–15]. With its well-established antioxidant and anti-inflammatory potentials [16–18], its use in OSF has also been evaluated by various studies. But due to inadequate comprehensive analysis of its effect in OSF, this systematic review was planned to analyse the efficacy of Spirulina in management of Oral Submucous Fibrosis.
Methods
Protocol and registration
The present systematic review was registered at the National Institute for Health Research PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD42020189399). This research protocol was designed according to the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) guidelines 2009 [19]. SWiM (Synthesis Without Meta-analysis) guidelines were intended to be followed in the absence of meta-analysis [20].
Eligibility criteria
Eligibility criteria for inclusion and exclusion of the studies in regards to Participants, Intervention, Comparator and Outcomes (PICO) was as shown in Table I.
Eligibility criteria of included studies according to Participants, Intervention, Comparator and Outcomes (PICO).
Search
Databases (MEDLINE via PubMed, Cochrane, Scopus, Science Direct, EBSCO-host, Clinical Trial Registry- India (CTRI) and Google scholar), review articles, bibliographies and related journal were searched from 1st January 2010 to 30th May 2020 by using various combinations MeSH terms and keywords describes below. This 10 years time span was selected after performing a pilot search which revealed lack of studies in literature before 10 years.
Disease − Oral submucous fibrosis, OSMF, Juxta epithelial fibrosis, Asian sideropenic dysphagia, OSF Intervention − Spirulina, Arthrospira maxima, spirulina maxima, seaweed algae, All − trans spirulina, All − cis spirulina.
All the articles available in the English language were included.
Study selection
Titles and/or abstracts of studies retrieved using the search strategy and those from additional sources were screened independently by two review authors (A and C). The full text of these potentially eligible studies was retrieved and independently assessed for eligibility by two review team members (A and C). Any disagreement between them over the eligibility of particular studies was resolved through discussion with a third reviewer (B). Authors and editors were communicated for retrieving full-text of the eligible articles.
Data collection process
Data collection was performed using a customized data extraction form that used following prefixes:
Title of the study.
Author's name.
Duration of study.
Year of publication.
Study setting.
Study design.
Study population details.
Method of randomization used (if any).
Details of intervention.
Details of comparator.
Indicators of acceptability of users.
Outcomes (primary & secondary).
Adverse effect and side effects.
Conclusion.
Risk of bias in individual studies
To evaluate the risk of bias in individual studies, different tools were used for randomized controlled trials (RCTs) and non- randomized controlled trials (NRCT). Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) was used for RCTs [21] and Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) was used for non-randomized controlled trails [22].
Synthesis of results
The result was provided for the findings from the included studies, focusing on intervention details (dose, type), characteristics of participants (age, gender,), type of outcome (primary, secondary, side effects etc.) and summaries of intervention effects for studies were provided by calculating risk ratio (for dichotomous outcomes) or standardized mean difference (for continuous outcomes). Heterogeneity of previously mentioned characteristics was assessed using chi square test (significance 0.1) and I2 statistics. Meta-analysis, if possible, would be formulated.
Results
Literature search and study selection
Figure 1 shows the study search process conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Database and hand searches of the reference list yielded 226 articles. After duplicate removal 162 articles were obtained. After screening title and abstract of articles,155 studies were excluded. The full-text of remaining 7 articles were screened which excluded 2 studies, as these two studies did not include the comparator groups. Finally, five studies were included for systematic review.
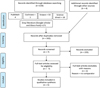 |
Fig. 1 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram. |
General characteristics of the included studies
The general characteristics of included studies are presented in Table II for four randomized controlled trials [23–26] and one non-randomized controlled trial [27]. A total of 240 subjects participated in the studies. All the studies were conducted in India except one which was conducted in Saudi Arabia [25]. All of the reported investigations were hospital/institution based and none were conducted in community settings. The average age of participants ranged from 18 to 56 years. Most studies showed male dominance except one study in which no information about gender was provided. Table II highlights the details of population, intervention and control or comparator of the included studies. In all five studies [23–27], diagnosis of Oral Submucous Fibrosis was based on clinical findings which was confirmed by histopathological investigation. Only one study mentioned about the clinical staging of OSF that used classification system by Ranganathan et al. [23] Enrolled population had a wide spectrum of OSF (i.e., from early to advanced), except one study [27] which included only Stage 1 and stage 2 of OSF.
Details of population, study groups and outcome (PICO) of included studies.
Formulation
Two studies [23,27] used a dosage of 500 mg twice daily whereas three studies [24–26] used a dosage 500 mg in two divided doses (250 mg BD). In three studies [24–26]. Spirulina was given alone, whereas in other two studies [23,27] it was given as an adjuvant therapy. In one study it was combined with mouth opening exercises [23] and in another study with intralesional steroid injection (Betamethasone 4 mg/ml). [27] Comparator groups showed variability amongst studies [23–27] (Tab. II). All studies were conducted for a period of 3 months with intermediate visits. During each visit all parameters were checked and recorded. The post-treatment follow-ups were carried out in three studies for a period of 2 months in two studies [24,25] and for 3 months in one study [26].
Clinical parameters
Mouth opening and burning sensation were analysed as primary outcome in all five studies [23–27]. Other outcomes which were analysed are—tongue protrusion, reduction in ulcers/erosions/vesicles, reduction in pain associated with lesion. Three studies [23,24,27] used vernier caliper for measuring mouth opening whereas, two studies [25,26] did not mention about measurement tool. For burning sensation and pain associated with lesion Visual Analogue Scale (VAS) were used as a measurement tool. Table II shows the details of outcomes for included studies.
Main outcomes
Mouth opening
Three studies by Patil et al. [24–26] used spirulina as a substitute therapy. Out of which one study showed statistically significant difference in mouth opening with better results in spirulina group as compared to Aloe vera group [25]. Whereas, other two studies showed statistically significant results in lycopene and oxitard group compared to spirulina group [24,26].
In the remaining two studies spirulina was used as an adjuvant therapy along with isometric exercises and intralesional steroid injections [23,27]. Study conducted by Shetty et al., which combined spirulina with intralesional steroid injection, showed highly significant improvement in mouth opening in spirulina group as compared to placebo [27]. Another combination study by Mulk et al., which combined spirulina with isometric exercises, showed statistically non-significant difference between spirulina group and pentoxyfilline.
Burning sensation
Two studies by Mulk et al. [23] and Shetty et al. [27] showed statistically significant results in reducing burning sensation in spirulina group as compared to pentoxifylline and placebo respectively. Whereas studies by Patil et al. [24–26], showed statistically non-significant difference in spirulina group as compared to lycopene, aloe vera and oxitard [24–26].
Other outcomes
Tongue protrusion
This outcome was evaluated in two studies by Mulk et al. [23] and Patil et al. [26]. Former study showed statistically significant results in spirulina group as compared to pentoxifylline. Whereas, later one showed statistically significant results in oxitard group as compared to spirulina.
Ulcers, erosions and vesicles
Three studies by Patil et al. [18–20] evaluated this parameter which showed statistically significant results for reduction in ulcers, erosions and vesicles in spirulina group as compared to lycopene, aloe vera and oxitard groups respectively.
Pain associated with lesion
This parameter was evaluated in two studies by Patil et al. which showed statistically non-significant difference in both the studies [24,25].
Adverse effects
Four studies [23–26] reported no adverse effects in spirulina group whereas one study [21] did not mention about side effects.
Quality of included studies
In this review, quality assessment was done for each study (Figs. 2 and 3). For Randomized controlled trials, Cochrane Tool of Risk of Bias (RoB2) was used (Fig. 2). The four RCT studies showed high risk of bias. This high risk of bias was due to inadequate information about randomization process, lack of allocation concealment and blinding. Similarly risk of bias assessment for non-randomized studies was done with ROBINS-I tool (Fig. 3) and the study was found to have serious risk of bias because of inadequate measurement of outcomes.
 |
Fig. 2 Risk of bias assessment for randomized controlled trial done by Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) tool. |
 |
Fig. 3 Risk of bias assessment for non-randomized control trial done by ROBINS-I tool. |
Meta-analysis
There was limited scope for meta-analysis because of the range of different comparators measured across the small number of existing trials as seen by grouping studies into categorical model.
Discussion
Oral Submucous Fibrosis (OSF) is a potentially malignant disease and it shows increased prevalence in India [6,7]. This high prevalence is attributed to widespread marketing of commercial tobacco and areca nut products. It is estimated that areca nut is consumed by 10–20% of the World's population [6]. Although the disease is observed in 11–60 year old individuals, occurrence in children is not rare with cases of 2.5–10 year old children being reported [28–30].
Patients with OSF mainly complains of two problems − an inability to open mouth which impedes function and burning sensation which restricts the intake of spicy food. Management is usually based on severity of disease and focuses on alleviating the signs and symptoms. Two recent systematic reviews failed to show evidence for effectiveness of any specific treatment for treating OSF [11,12]. This may be due to lack of standardisation in the study designs and paucity of data available. So, no single regime has proved to be entirely satisfactory.
A chronic disease like OSF might require treatment for longer time depending on the severity of symptoms. Therefore, considering natural therapies which are mostly safe could probably benefit such patients. Spirulina, a blue green alga have been studied in OSF (and many other conditions) due to its various beneficial effects. It contains β-carotene, carotenoids and phycocyanin which are believed to have antioxidant and anti-inflammatory activity. Phycocyanin in spirulina inhibits proinflammatory cytokine formation, suppresses cyclooxygeanase-2 (COX-2) expression and decreases prostaglandin E2 production [18]. It also scavenges free radicals, including alkoxyl, hydroxyl and peroxyl radicals thus exhibiting its antioxidant activity. Another component of spirulina, β-carotene inhibits the production of nitric oxide and prostaglandin E2. Thus, producing an anti-inflammatory activity. This could be beneficial in OSF, in which there is upregulation of various cytokines. Additionally, increased oxidative stress in these patients due to generation of reactive oxygen species (ROS) that causes activation of NF- kappa B, JNK and p38 stimulated by Arecoline, could be benefited by the potential antioxidant activity of carotenoids and phycocyanin components of spirulina [18].
Spirulina is useful in various diseases like nutritional deficiencies (Vit A, iron), cardiac diseases, diabetes mellitus, dyslipidaemia, inflammatory and degenerative diseases, hepatocellular carcinoma and breast cancer [31,32]. Some recent systematic reviews reported the beneficial effects of spirulina supplementations on plasma lipid concentration [33], obesity [34], human health [13], glycaemic control and serum lipoproteins [14,15]. In dentistry, apart from its use in OSF, it has been tried in treatment of periodontitis (topically in gel form) [35], reducing dental plaque and gingivitis (topical as 0.5% mouthwash) [36] and in oral mucosal lesions like leukoplakia [37,38]. Spirulina extracts have also been shown to inhibit DMBA (7,12 dimethylbenz(a)-anthracene) induced epidermoid carcinoma of buccal pouch in animal studies by stimulating TNF-α positive cells and subsequently causing tumour regression [39–41].
With regard to dosage, a systematic review reported that spirulina consumption for a period varying from 1 to 12 months, with doses ranging from 0.5 to 20 g day−1 showed significant benefits in various conditions [13]. These studies used spirulina systemically, but topically in the form of gel [35] and mouthwash [36] had also been tried. In the present review, two studies [23,27] used a dosage of 500 mg twice daily whereas three studies [24–26] used a dosage 500 mg in two divided doses (250 mg BD).
All five included studies reported that spirulina is an effective herbal medicine in improvement of mouth opening, reducing burning sensation and lesions like ulcers, erosions or vesicles. In three studies spirulina was compared with single intervention like lycopene [24], aloe vera [25] and oxitard [26]. When compared with lycopene and oxitard, spirulina was found to be less effective in improving mouth opening, in contrast to aloe vera in which spirulina was more effective [24–26]. Spirulina group also demonstrated better results for reducing lesions when compared with these three interventions [24–26]. Whereas, for burning sensation, all were equally effective [24–26].
Other two studies used combination therapy. In one study spirulina along with isometric exercises was compared with pentoxifylline combined with isometric exercises [23] while in another study spirulina along with intralesional steroids was compared with placebo combined with intralesional steroid (betamethasone) injection [27]. When compared with pentoxifylline spirulina proved to be equally effective in improving mouth opening [23]. However, spirulina showed better results on combining it with intralesional steroids [27]. For alleviating burning sensation spirulina group showed significant outcomes as stated by these two studies [23,27].
In this review, all enrolled studies showed that spirulina is a safe, well-tolerated and without any adverse effects. Lycopene, aloe vera and pentoxifylline have well established antioxidant and anti-inflammatory potential [42–44]. Spirulina showed efficacy somewhat similar to them. Thus, it could be inferred that although spirulina has been evaluated as an effective herbal medicine in improvement of mouth opening and reducing burning sensation, the high risk of bias associated with all the studies warrant cautious interpretation of its precise effect as a substitute or adjuvant therapy compared to other interventions or along with other interventions respectively, mainly due to the heterogenous data available.
In all included studies spirulina was used as a supplementary treatment modality for OSF. Dietary Supplements Information Expert Committee (DSI-EC) of the United States Pharmacopeial Convention (USP) assigned it, a class A safety rating [45]. Considering its safety and beneficial effects in health and diseases, spirulina could probably be considered as a dietary biomass in the management of OSF.
Limitations and future scope
The main limitations of the present review are limited number of studies with considerable heterogeneity and high risk of bias. This high risk of bias is because of inadequate information about randomization process, lack of allocation concealment and lack of blinding. Therefore, to exactly define the efficacy of spirulina, more number of studies with more standard and uniform trials on larger population with different regimens of spirulina should be carried.
Conclusion
Management of OSF has been a challenge always. Newer drugs have been emerging continuously for the treatment of this disease. When considering natural therapies mainly due to their safety concerns, spirulina needs to be evaluated further in OSF due to the weak evidence obtained in the present systematic review, regarding the effectiveness of spirulina in treating OSF. Thus, well-designed and standard clinical trials are still required to accurately provide the evidence of effectiveness of spirulina as compared to other interventions in the management of OSMF.
Authors contribution
Dr. Rashmi Kulkarni: Conceptualization, Methodology, Writing- Original draft. Dr. Ashita Kalaskar: Supervision, Validation, review editing. Dr. Neha Gupta: Writing - Review & Editing. Dr. Ritesh Kalaskar: Validation & editing.
Conflicts of interest
The authors declare that there is no conflict of interest.
Funding
This research did not receive any specific funding.
Ethical approval
Ethical approval was not required.
Informed consent
This article does not contain any studies involving human subjects.
References
- Rai A, Siddiqui M, Parveen S, Parveen S, Rasheed A, Ali S. Molecular pathogenesis of oral submucous fibrosis: a critical appraisal. Biomed Pharmacol J 2019;12:2027–2036. [CrossRef] [Google Scholar]
- Pindborg JJ, Sirsat SM. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol 1966;22:764–779. [CrossRef] [PubMed] [Google Scholar]
- Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 2007;36:575–580. [CrossRef] [PubMed] [Google Scholar]
- More CB, Rao NR. Proposed clinical definition for oral submucous fibrosis. J Oral Biol Craniofac Res 2019;9:311–314. [CrossRef] [PubMed] [Google Scholar]
- Khan S, Sinha A, Kumar S, Iqbal H. Oral submucous fibrosis: current concepts on aetiology and management − a review. J Indian Acad Oral Med Radiol 2018;30:407–407. [CrossRef] [Google Scholar]
- Rao NR, Villa A, More CB, Jayasinghe RD, Kerr AR, Johnson NW. Oral submucous fibrosis: a contemporary narrative review with a proposed inter-professional approach for an early diagnosis and clinical management. J Otolaryngol Head Neck Surg 2020;49:3. [CrossRef] [PubMed] [Google Scholar]
- Chattopadhyay A, Ray JG. Molecular Pathology of malignant transformation of oral submucous fibrosis. JEP(T) 2016;35. Available from: http://www.dl.begellhouse.com/journals/0ff459a57a4c08d0,2d1506ed13a32809,0c3016997dd60aeb.html [Google Scholar]
- Arakeri G, Patil SG, Aljabab AS, Lin K-C, Merkx MaW, Gao S, et al. Oral submucous fibrosis: an update on pathophysiology of malignant transformation. J Oral Pathol Med 2017;46:413–417. [CrossRef] [PubMed] [Google Scholar]
- Srivastava R, Jyoti B, Pradhan D, Siddiqui Z. Prevalence of oral submucous fibrosis in patients visiting dental OPD of a dental college in Kanpur: a demographic study. J Family Med Prim Care 2019;8:2612–2617. [CrossRef] [PubMed] [Google Scholar]
- Teh MT, Tilakaratne WM, Chaplin T, Young BD, Ariyawardana A, Pitiyage G, et al. Fingerprinting genomic instability in oral submucous fibrosis. J Oral Pathol Med 2008;37:430–436. [CrossRef] [PubMed] [Google Scholar]
- More CB, Jatti Patil D, Rao NR. Medicinal management of oral submucous fibrosis in the past decade − a systematic review. J Oral Biol Craniofac Res 2020;10:552–568. [CrossRef] [PubMed] [Google Scholar]
- Fedorowicz Z, Chan Shih-Yen E, Dorri M, Nasser M, Newton T, Shi L. Interventions for the management of oral submucous fibrosis. Cochrane Database Syst Rev 2008;CD007156. [PubMed] [Google Scholar]
- de la Jara A, Ruano-Rodriguez C, Polifrone M, Assunçao P, Brito-Casillas Y, Wägner AM, et al. Impact of dietary Arthrospira (Spirulina) biomass consumption on human health: main health targets and systematic review. J Appl Phycol 2018;30:2403–2423. [CrossRef] [Google Scholar]
- Hamedifard Z, Milajerdi A, Reiner Ž, Taghizadeh M, Kolahdooz F, Asemi Z. The effects of spirulina on glycemic control and serum lipoproteins in patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Phytother Res 2019;33:2609–2621. [CrossRef] [PubMed] [Google Scholar]
- Hatami E, Ghalishourani S-S, Najafgholizadeh A, Pourmasoumi M, Hadi A, Clark CCT, et al. The effect of spirulina on type 2 diabetes: a systematic review and meta-analysis. J Diabetes Metab Disord 2021. Available from: https://doi.org/10.1007/s40200-021-00760-z [PubMed] [Google Scholar]
- Finamore A, Palmery M, Bensehaila S, Peluso I. Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly spirulina. Oxidat Med Cell Longev 2017;2017:3247528. [CrossRef] [Google Scholar]
- Ravi M, De SL, Azharuddin S, Paul SF. The beneficial effects of Spirulina focusing on its immunomodulatory and antioxidant properties. Nutr Diet Suppl 2010;2:73–83. [Google Scholar]
- Deng R, Chow T-J. Hypolipidemic, antioxidant and antiinflammatory activities of microalgae spirulina. Cardiovasc Ther 2010;28:e33–45. [CrossRef] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [CrossRef] [PubMed] [Google Scholar]
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. [CrossRef] [PubMed] [Google Scholar]
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366. Available from: https://www.bmj.com/content/366/bmj.l4898 [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. Available from: https://www.bmj.com/content/355/bmj.i4919 [Google Scholar]
- Mulk BS, Deshpande P, Velpula N, Chappidi V, Chintamaneni RL, Goyal S. Spirulina and pentoxyfilline–A novel approach for treatment of oral submucous fibrosis. Journal of clinical and diagnostic research: JCDR 2013;7:3048. [Google Scholar]
- Patil S, Khandelwal S, Maheshwari S. Comparative efficacy of newer antioxidants spirulina and lycopene for the treatment of oral submucous fibrosis. Clin Cancer Invest J 2014;3:482. [CrossRef] [Google Scholar]
- Patil S, Al-Zarea BK, Maheshwari S, Sahu R. Comparative evaluation of natural antioxidants spirulina and aloe vera for the treatment of oral submucous fibrosis. J Oral Biol Craniof Res. 2015;5:11–5. [CrossRef] [Google Scholar]
- Patil SR, Maragathavalli G, Ramesh D, Munishekar MS, Rao KA, Alam MK. Comparative efficacy of newer antioxidants spirulina and oxitard for the treatment of oral submucous fibrosis. Int Med J 2019;26. [Google Scholar]
- Shetty P, Shenai P, Chatra L, Rao P. Efficacy of spirulina as an antioxidant adjuvant to corticosteroid injection in management of oral submucous fibrosis. Indian J Dental Res 2013;24:347–350. [CrossRef] [PubMed] [Google Scholar]
- Jain A, Taneja S. Oral submucous fibrosis in pediatric patients: a systematic review and protocol for management. Int J Surg Oncol 2019;2019. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6463605/ [Google Scholar]
- Shah B, Lewis MA, Bedi R. Oral submucous fibrosis in a 11-year-old Bangladeshi girl living in the United Kingdom. Br Dent J 2001;191:130–132. [CrossRef] [PubMed] [Google Scholar]
- Hayes PA. Oral submucous fibrosis in a 4-year-old girl. Oral Surg Oral Med Oral Pathol 1985;59:475–478. [CrossRef] [PubMed] [Google Scholar]
- Mahmoud YI, Shehata AMM, Fares NH, Mahmoud AA. Spirulina inhibits hepatocellular carcinoma through activating p53 and apoptosis and suppressing oxidative stress and angiogenesis. Life Sciences 2021;265:118827. [CrossRef] [PubMed] [Google Scholar]
- Najem AM, Abed IJ, Fadhel LZ. Assessment the activity of spirulina platensis alcoholic extract in MCF-7 breast cancer cells inhibition and P-53 gene induction. Public Health 8. [Google Scholar]
- Serban M-C., Sahebkar A, Dragan S, Stoichescu-Hogea G, Ursoniu S, Andrica F, et al. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin Nutr 2016;35:842–851. [CrossRef] [PubMed] [Google Scholar]
- Moradi S, Ziaei R, Foshati S, Mohammadi H, Nachvak SM, Rouhani MH. Effects of Spirulina supplementation on obesity: a systematic review and meta-analysis of randomized clinical trials. Complement Ther Med 2019;47:102211. [CrossRef] [PubMed] [Google Scholar]
- Mahendra J, Mahendra L, Muthu J, John L, Romanos GE. Clinical effects of subgingivally delivered spirulina gel in chronic periodontitis cases: a placebo controlled clinical trial. J Clin Diagn Res 2013;7:2330–2333. [PubMed] [Google Scholar]
- Maniyar R. Effectiveness of spirulina mouthwash on reduction of dental plaque and gingivitis: a clinical study. Int J Pharm Pharmaceut Sci 2017;9:136. [CrossRef] [Google Scholar]
- Mathew B, Sankaranarayanan R, Nair P, Varghese C, Somanathan T, Amma B, et al. Evaluation of chemoprevention of oral cancer with Spirulina fusiformis. Nutr Cancer 1995;24:197–202. [CrossRef] [PubMed] [Google Scholar]
- Karkos PD, Leong SC, Karkos CD, Sivaji N, Assimakopoulos DA. Spirulina in clinical practice: evidence-based human applications. Evidence-Based Complemen Alternat Med 2010;2011:enen058. [Google Scholar]
- Schwartz J, Shklar G, Reid S, Trickier D. Prevention of experimental oral cancer by extracts of Spirulina-Dunaliella algae. Nutr Cancer 1988;11:127–34. [CrossRef] [PubMed] [Google Scholar]
- Shklar G, Schwartz J. Tumor necrosis factor in experimental cancer regression with alphatocopherol, beta-carotene, canthaxanthin and algae extract. Eur J Cancer Clin Oncol 1988;24:839–850. [CrossRef] [PubMed] [Google Scholar]
- Schwartz J, Shklar G. Regression of experimental hamster cancer by beta carotene and algae extracts. J Oral Maxillofac Surg 1987;45:510–515. [CrossRef] [PubMed] [Google Scholar]
- Gupta N, Kalaskar A, Kalaskar R. Efficacy of lycopene in management of oral submucous fibrosis − a systematic review and meta-analysis. J Oral Biol Craniofac Res 2020;10:690–697. [CrossRef] [PubMed] [Google Scholar]
- Al-Maweri SA, Ashraf S, Lingam AS, Alqutaibi A, Abdulrab S, Alaizari N, et al. Aloe vera in treatment of oral submucous fibrosis: a systematic review and meta-analysis. J Oral Pathol Med 2019;48:99–107. [CrossRef] [PubMed] [Google Scholar]
- Liu J, Chen F, Wei Z, Qiu M, Li Z, Hongxia D, et al. Evaluating the efficacy of pentoxifylline in the treatment of oral submucous fibrosis: a meta-analysis. Oral Diseases 2017;24. [Google Scholar]
- Marles RJ, Barrett ML, Barnes J, Chavez ML, Gardiner P, Ko R, et al. United States pharmacopeia safety evaluation of spirulina. Crit Rev Food Sci Nutr 2011;51:593–604. [CrossRef] [PubMed] [Google Scholar]
All Tables
Eligibility criteria of included studies according to Participants, Intervention, Comparator and Outcomes (PICO).
All Figures
 |
Fig. 1 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram. |
| In the text | |
 |
Fig. 2 Risk of bias assessment for randomized controlled trial done by Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) tool. |
| In the text | |
 |
Fig. 3 Risk of bias assessment for non-randomized control trial done by ROBINS-I tool. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


