| Issue |
J Oral Med Oral Surg
Volume 27, Number 4, 2021
|
|
|---|---|---|
| Article Number | 46 | |
| Number of page(s) | 4 | |
| DOI | https://doi.org/10.1051/mbcb/2021036 | |
| Published online | 15 October 2021 | |
Case Report
Diagnosis of a plasmoblastic lymphoma of the mandible after renal transplantation: a case report
Service d'odontologie, chirurgie orale et implantologie, CHU de Rennes, rue Henri le Guilloux, 35000 Rennes, France
* Correspondence: legeard.ines@gmail.com
Received:
4
January
2021
Accepted:
1
September
2021
Introduction: Post-transplant lymphoproliferations (PTL) are a severe complication of solid organ transplants. Their locations can be extra-nodal. Observation: The diagnosis and management of a non-Hodgkin's plasmablastic lymphoma of mandibular localization affecting a 66-year-old kidney transplanted patient are reported here. Comment: The main risk factors for non-Hodgkin lymphoma are immunosuppression and infection with Epstein-Barr virus. Clinical and radiographic examinations, which are not specific, must be supplemented by a histological examination. Treatment which is not consensual will most often consist of a reduction in immunosuppression coupled with chemotherapy. Conclusion: Despite a constant evolution in the incidence and clinical picture of post-transplant lymphomas, the role of the dentist remains essential in the early detection of lesions.
Key words: Post-transplant lymphoproliferation / Epstein-Barr virus / oral cavity
© The authors, 2021
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
According to the new classification from the World Health Organization, post-transplantation lymphoproliferative (PTL) syndromes cover a heterogeneous set of benign or malignant pathologies, including lymphoma, occurring in a context of post-transplantation immunosuppression due to quantitative or qualitative T lymphocyte damage [1]. They represent a severe complication occurring in patients with solid organ transplants such as kidneys.
Non-Hodgkin lymphoma (NHL) are usually lymph node, but 20–30% of the sites are extra-nodal [2], particularly affecting the oral cavity. A case of mandibulare plasmablastic lymphoproliferative syndrome in an Epstein-Barr virus (EBV) positive patient is presented here.
Since 2011, only one case of oral cavity plasmablastic lymphoma occurring after solid organ transplantation has been documented.
Observation
A 66-year-old man presented to the dental emergency department following the appearance of buccal and lingual swelling in the lower right mandibular molar region evolving over the past two weeks. This patient had a kidney transplant 4 years ago. His treatment was tacrolimus, cortisone and catapressan.
Intraoral examination revealed vestibular and lingual gingival hypertrophy ranging from 44 to 47 (increased around 46, mesioversed) the mucosa was erythematous and ulcerated (Fig. 1). This lesion was painless on palpation. The teeth facing the lesion were vital. Neurological examination of the right alveolar nerve territory was normal.
The orthopantomogram showed a periodontal pocket mesial to tooth 46 (Fig. 2). The cone-beam was unremarkable (Fig. 3).
Due to the suspicious macroscopic appearance of the lesion, a biopsy was performed at the site. Histological analysis was in favor of a post-transplant monomorphic lymphoproliferative syndrome. The immunohistochemistry specified a plasmablastic-type lymphoma.
The patient was referred to the hematology department for an extension assessment. The whole body CT scan did not show any other localization.
PCR was positive for EBV with 132,000 copies of the viral genome.
The strictly medical management consisted of treatment with 2 courses of R-CHOP (R-CHOP: rituximab, cyclophosphamide, vincristine, doxorubicin, methylprednisolone) associated with a reduction of the dosage of tacrolimus, in order to reduce immunosuppression induced by this treatment (from 3 mg a day to 2 mg a day).
The response to treatment was favorable and complete remission was obtained after this treatment.
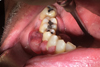 |
Fig. 1 Intraoral picture: vestibular and lingual gingival hypertrophy ranging from 44 to 47. |
 |
Fig. 2 Orthopantomogram: periodontal pocket mesial to tooth 46. |
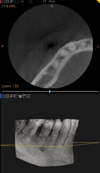 |
Fig. 3 Computed tomography focus on sector 4: unremarkable. |
Comments
In terminal chronic kidney failure, kidney transplantation is currently the standard treatment ensuring better patient survival.
However, immunosuppressive anti-rejection therapy greatly increases the risk of developing infections and neoplasms. Thus, cancers occur in 30% of cases and represent the major cause of death in kidney transplant patients. After skin cancer, malignant lymphoid proliferation is the most common [3].
The new classification from the World Health Organization groups NHL, myeloma and Hodgkin lymphoma after transplant into a category called post-transplant monomorphic lymphoproliferations [1].
NHL represents, after squamous cell carcinoma, the second cancer of the head and neck region, accounting for 2.6% of the lesions encountered [4]. After renal transplantation, its incidence is 0.4% at one year and 1% at five years [5].
The main risk factors for developing PTL are infection with EBV and immunosuppression [2]. In fact, in a transplant patient, the antiviral defenses are impaired mainly by the immunosuppressive treatment which decreases the cytotoxic T response, responsible for the fight against cells infected with EBV.
EBV contamination plays a major role in this oncogenic process. This lymphotropic virus infects over 90% of the general population. Unlike an immunocompetent patient, the transplant patient does not have sufficient cellular immunity to control his primary infection and periodic reactivations of EBV. An EBV-negative patient (8% of the general population) receiving a transplant from an EBV-positive donor has a high risk of developing post-transplant lymphoma: 11.5% after primary infection and 3.6% after reactivation [6].
The locations of NHL are varied. The main sites are the graft itself (12%), digestive (25%), cerebral (14%), isolated ganglia (13%) but also cutaneous, ENT and oral [5]. In the oral sphere, they can involve the tongue, the gingiva, the hard palate, the jaws, the palatine and lingual tonsils, and more generally all the structures constituting the Waldeyer's ring [7].
Among NHL, the most frequently encountered histological type is that with large B cells [8].
Plasmablastic lymphoma (PBL) is a rare aggressive variant of large B cell lymphoma defined as a proliferation of large neoplastic plasmoblasts [9].
PBLs in immunocompromised patients, like transplanted [10] or HIV-positive patients, show a predilection for the oral cavity, although extra-oral involvement is found [11]. They are predominant in male and more frequently affect the adult population, with a median age of presentation being in the fourth decade [12,13].
PBLs also develop in immunocompetent patients, usually already over 60 years old by the time of showing.
A third of these PBLs' occurrences are in absence of EBV+ [14].
Clinically, in our patient, the swelling was not painful but we can also find bone or dental pain when lymphoid cells infiltrate the dental pulp. The clinical picture can also find dental mobility or loss, pathological fractures [1,15] or even paresthesia of the inferior alveolar nerve secondary to its compression or infiltration. A general sign such as fever, night sweats or weight loss may also appear [12].
Radiographic examination is not specific in the initial stage. At a more advanced stage, we may find osteolysis, periosteal thickening, periodontal enlargement or enlargement of the mandibular canal [1,16].
Due to the absence of radio-clinical specificity, several diagnostic hypotheses can be raised: either benign (periodontal disease, pericoronitis, periodontal abscess), or malignant (multiple myeloma [12], primary bone carcinoma, bone metastasis, osteomyelitis).
The diagnosis must be confirmed by histological and immunohistochemical analysis.
The detection of EBV is conventionally carried out by in situ hybridization (search for RNA not coding for EBV) or by immunohistochemistry (detection of EBV Ag or proteins expressed by EBV).
In our patient, this test was positive and confirmed by PCR test with a high replication rate, indicating a recent reactivation of the virus.
Treatment can be preemptive and regular monitoring of EBV viral loads is sometimes undertaken to treat primary infection or reactivation with EBV [17].
When an LPT is proven, the curative treatment will be assessed in a multidisciplinary discussion on criteria such as the histological subtype, but also the location and size of the tumor. There are currently no standardized approaches, as therapeutic tools and indications are still poorly established.
A reduction in immunosuppression is often sought as a first-line treatment, but it is most often insufficient and must be combined with chemotherapy, immunotherapy, immunochemotherapy or cell therapy, or even in more rare cases with surgery and radiotherapy when the tumor remains localized to the original site [18].
Chemotherapy consists of a combination of chemotherapeutic agents such as CHOP alone (cyclophosphamide, doxorubicin, vincristine, prednisone) [19] or, as for the patient here, in combination with rituximab, which is a monoclonal antibody making it possible to strengthen or restore a competent immune system. More recently, therapies specifically targeting proteins located on the surface of cancer cells have been introduced to stop their growth and spread [20].
The initial response to chemotherapy is favorable in PBL with an overall response rate of up to 77% [12]. The prognosis without chemotherapy is poor, with a median survival of 3 months [12].
Conclusion
Despite a constant change in the incidence and clinical picture of PTL, as well as an improvement in their management, these blood disorders still represent a serious complication of transplants.
A better understanding of the risk factors and proliferative mechanisms inherent in PTL will improve the prevention and treatment of these hemopathies.
In this context, the role of the dentist remains essential in the early detection of oral lesions that could be related to extra- nodal PTL.
Authors contribution
I. LEGEARD: rédaction CHEVROLLIER M-A: révision BADER G.: révision.
Conflicts of interests
The authors declare that they have no conflicts of interest in relation to this article.
Informed consent
The authors declare declare that informed consent not required.
Ethical commitee approval
The authors declare that Ethical approval not required.
Source of fouding
This research did not receive any specific funding.
References
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375–2390. [CrossRef] [PubMed] [Google Scholar]
- Wong GB, Spadafora S, Barbon N, Caputo M. Primary extranodal B-cell non-Hodgkin lymphoma mimicking an endodontic lesion: report of 2 cases. J Can Dent Assoc 2013;79:d93. [PubMed] [Google Scholar]
- Caillard S, Olagne J, Gautier Vargas G, Cognard N, Muller C, Moulin B. Lymphoproliférations survenant après transplantation d'organes solides. EMC − Néphrologie 2017;14:1–11 [Article 18-065-D-20]. [Google Scholar]
- Batra R, Kaur H, Jindal S. Extranodal large B-cell type aggressive non-Hodgkin's lymphoma. Indian J Dent 2014;5:225–8. [CrossRef] [PubMed] [Google Scholar]
- Caillard S, Lamy FX, Quelen C, Dantal J, Lebranchu Y, Lang P, et al. Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas. Am J Transplant 2012;12:682–693. [CrossRef] [PubMed] [Google Scholar]
- Ho M, Miller G, Atchison RW, Breinig MK, Dummer JS, Andiman W, et al. Epstein-Barr virus infections and DNA hybridization studies in posttransplantation lymphoma and lymphoproliferative lesions: the role of primary infection. J Infect Dis 1985;152:876–886. [CrossRef] [PubMed] [Google Scholar]
- Vega F, Lin P, Medeiros LJ. Extranodal lymphomas of the head and neck. Ann Diagn Pathol 2005;9:340–350. [CrossRef] [PubMed] [Google Scholar]
- Souto GR, Pereira TS, Castro AF, Mesquita RA. Diffuse large B-cell lymphoma, not otherwise specified of the palate: a case report. J Clin Exp Dent 2013;5:e287–e290. [CrossRef] [PubMed] [Google Scholar]
- Castillo JJ, Beltran BE, Miranda RN, Young KH, Chavez JC, Sotomayor EM. EBV-positive diffuse large B-cell lymphoma of the elderly: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016;91:529–537. [CrossRef] [PubMed] [Google Scholar]
- Payet X, Raybaud H, Kipper M, Fino E, Voha C. Lymphome de la lèvre après transplantation rénale : à propos d'un cas. J Oral Med Oral Surg 2020;26:2. [CrossRef] [EDP Sciences] [Google Scholar]
- Carbone A, Gaidano G, Gloghini A, Ferlito A, Rinaldo A, Stein H. AIDS-related plasmablastic lymphomas of the oral cavity and jaws: a diagnostic dilemma. Ann Otol Rhinol Laryngol 1999;108:95–99. [CrossRef] [PubMed] [Google Scholar]
- Hansra D, Montague N, Stefanovic A, Akunyili I, Harzand A, Natkunam Y, et al. Oral and extraoral plasmablastic lymphoma: similarities and differences in clinicopathologic characteristics. Am J Clin Pathol 2010;134:710–719. [CrossRef] [PubMed] [Google Scholar]
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood 2015;125:2323–2330. [CrossRef] [PubMed] [Google Scholar]
- Morscio J, Dierickx D, Nijs J, et al. Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and posttransplant patients: single-center series of 25 cases and meta-analysis of 277 reported cases. Am J Surg Pathol 2014;38:875–886. [CrossRef] [PubMed] [Google Scholar]
- Parihar S, Garg RK, Narain P. Primary extra-nodal non-Hodgkin's lymphoma of gingiva: a diagnostic dilemma. J Oral Maxillofac Pathol 2013;17:320. [PubMed] [Google Scholar]
- Yamada T, Kitagawa Y, Ogasawara T, Yamamoto S, Ishii Y, Urasaki Y. Enlargement of mandibular canal without hypesthesia caused by extranodal non-Hodgkin's lymphoma: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;89:388–92. [CrossRef] [PubMed] [Google Scholar]
- Bakker NA, Verschuuren EAM, Erasmus ME, Hepkema BG, Veeger NJGM, Kallenberg CGM, et al. Epstein-Barr virus-DNA load monitoring late after lung transplantation: a surrogate marker of the degree of immunosuppression and a safe guide to reduce immunosuppression. Transplantation 2007;83:433–438. [CrossRef] [PubMed] [Google Scholar]
- Patil AV, Deshpande RB, Kandalgaonkar SM, Gabhane MH. Diffuse large B-cell lymphoma (extranodal) of maxillary buccal vestibule. Journal of oral and maxillofacial pathology: JOMFP. 2015;19:270. [Google Scholar]
- Fohrer C, Caillard S, Koumarianou A, Ellero B, Woehl-Jaeglé M-L, Meyer C, et al. Long-term survival in post-transplant lymphoproliferative disorders with a dose-adjusted ACVBP regimen. Br J Haematol. 2006;134:602–612. [CrossRef] [PubMed] [Google Scholar]
- Gustafsson A, Levitsky V, Zou JZ, Frisan T, Dalianis T, Ljungman P, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood 2000;95:807–814. [CrossRef] [PubMed] [Google Scholar]
All Figures
 |
Fig. 1 Intraoral picture: vestibular and lingual gingival hypertrophy ranging from 44 to 47. |
| In the text | |
 |
Fig. 2 Orthopantomogram: periodontal pocket mesial to tooth 46. |
| In the text | |
 |
Fig. 3 Computed tomography focus on sector 4: unremarkable. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


