| Issue |
J Oral Med Oral Surg
Volume 29, Number 4, 2023
|
|
|---|---|---|
| Article Number | 37 | |
| Number of page(s) | 10 | |
| DOI | https://doi.org/10.1051/mbcb/2023041 | |
| Published online | 22 January 2024 | |
Original Research Article
Comparative evaluation of healing after surgical excision of oral mucosal lesions using PRF and collagen membrane
Department of Oral & Maxillofacial Surgery, Swami Devi Dyal Hospital & Dental College, Barwala - 134118, Panchkula, Haryana
* Correspondence: drvishalpo@gmail.com
Received:
19
February
2023
Accepted:
25
September
2023
Objectives The purpose of the comparative study was to evaluate the clinical parameters affecting healing after surgical excision of superficial, potentially malignant oral lesions using Platelet Rich Fibrin (PRF) and Collagen Membrane. Material and methods: A total of 100 patients requiring treatment for oral mucosal lesions (OML) were enrolled through a randomized selection of two different groups, where Group 1 (50 patients) received PRF while Group 2 (50 patients) received Collagen membrane following excisional biopsy. Parameters checked were Pain, Clinical Healing, Granulation Tissue, Epithelialization, Wound Contracture, and Complications postoperatively at 3rd, 7th and 30th day. Results: Group 1 showed better results with post-operative pain, clinical healing and granulation presence when compared to Group 2. Conclusion: PRF can be ascertained as a better dressing material than Collagen Membrane with better healing potential.
Key words: Clinical healing / Collagen membrane / PRF / Surgical excision
© The authors, 2023
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Oral Mucosal Lesions (OML) are defined as any abnormal change or any swelling on the oral mucosal surface. They are generally categorized as scrapable or non-scrapable and white or red lesions, which can be benign, premalignant or malignant in nature [1]. The increased incidence of oral potentially malignant disorders (OPMDs) and frank oral malignancies can be attributed to the habit of consumption of tobacco products in Indian subcontinent [2].
The term OPMD was introduced in 2007, following an expert group meeting coordinated by the WHO Collaborating Centre for Oral Cancer [3] and the terminology is now included in the 4th edition of the WHO Classification on Head and Neck Tumors [4]. WHO merged the term pre-cancerous lesions and pre-cancerous condition to represent all the clinical manifestations that carry a risk to oral cancer as “Oral Potentially Malignant Disorders” (OPMDs) [5]. Accordingly, World Health Organization defined OPMDs as “clinical presentations that carry a risk of cancer development in the oral cavity, whether in a clinically definable precursor lesion or in clinically normal oral mucosa” [6,7]. Characteristically, OPMDs present with diverse clinical attributes, such as color variations (white, red, and mixed white-red), morphological changes (plaque/plateau, smooth, grooved, wrinkled, granular, atrophic), and different sizes, involving different anatomical sites in the oral cavity [8]. The spectrum of OPMDs include oral leukoplakia, erythroplakia, erythro-leukoplakia, tobacco pouch keratosis, oral submucous fibrosis (OSF), palatal lesions in reverse smokers, oral lichen planus, oral lichenoid reactions, graft-versus-host disease (GvHD) [3], oral lupus erythematosus, and some hereditary conditions, such as dyskeratosis congenita and epidemolysis bullosa [9,10]. The natural history of OPMDs has no consistent pattern and it is difficult to predict who may develop cancer following the detection of an OPMD. A systematic review of observational studies on oral leukoplakia reported a malignant transformation rate ranging from 0.13–34.0% [11] and a meta-analysis of studies on oral lichen planus (including lichenoid reactions) reported a range from 0–3.5% [12]. The management of oral lesions in these disorders can be medical or surgical [13]. The commended protocol is complete surgical excision of the lesion and fibrotomy in cases of OSMF, with or without graft placement for definitive histological diagnosis and effective treatment irrespective of presence or absence of dysplasia [14].
Wound healing is a sequence of cellular and biochemical activities, aiming towards restoring tissue integrity and functional capacity after injury. After excision, primary closure is advocated for smaller wounds, while larger wounds if left uncovered, heal by secondary intension [15]. Thus, such large intraoral wounds should be covered with a dressing material or a graft to prevent microbial infection, foreign body contamination, wound contracture, and to improve healing. Autologous skin grafts, free mucosal grafts have been tried, but it results in donor site morbidity and have disadvantages in intraoral use like limited availability, presence of adnexal structures and abnormal tissue texture interfering with function [16]. Alloplastic materials, like hyperdry amniotic membrane and artificial dermis, have been used with satisfactory results, but have a risk of allergic reactions. Therefore, there is search for an ideal dressing material to cover these defects [17].
Autologous platelet rich fibrin (PRF) have been used as a dressing material and it has shown good results after reconstruction of oral mucosal defects post-excision of soft tissue premalignant lesions. Platelet-rich fibrin (PRF) is a second-generation platelet concentrate, developed in 2001 by Choukroun et al. [18] now defined as “an immune and platelet concentrate collected on a single fibrin membrane containing all the constituents of a blood sample favorable to healing and immunity”. PRF has certain clear advantages over other platelet concentrates, including simple, quick, single-staged fabrication, cost effectiveness, and no addition of bovine thrombin or anticoagulants, thus reducing the risk of antigenicity [19]. It is also shown that conversion of fibrinogen to fibrin in PRF is slow, with physiologically available thrombin of blood, creating a fibrin network similar to a natural one, leading to more efficient cell migration and proliferation. It was hence proposed that grafting of autologous PRF membrane after excision of superficial, nondysplastic, potentially malignant, oral lesions could help in wound coverage and might have a positive effect on wound healing and epithelialization [20,21].
One of the biologic products is bovine-derived xenogenous collagen, a biologic plastic, which can be molded like wax into desired forms. Because of its easy availability, method of extraction, purification, and low antigenicity, it has been used under many clinical conditions as a temporary dressing material with favorable results [22]. Collagen when used to cover the raw area provides the coverage for sensitive nerve endings thereby diminishing degree of pain [8,9]. Xenogenous collagen membrane have good conformability in the mucous lining, thus yielding a good clinical assessment with regard to its suppleness, resiliency and dressing ability to mimic oral wound and surrounding normal tissue.
However, there is no clear consensus in the literature, on whether platelet rich fibrin (PRF) or Collagen membrane provides a better wound cover and propagate healing. Hence, this study was undertaken to compare the healing and the clinical parameters affecting healing after surgical excision of superficial, potentially malignant oral lesions using PRF and collagen membrane as a dressing material.
Materials and methodology
Sample selection and study design
The comparative study was conducted on 100 patients, selected from those reporting to the Institutional Department of Oral and Maxillofacial Surgery requiring treatment for Superficial Oral Mucosal Lesions, in the period of August 6th, 2019 to November 5th, 2021. Initial patient screening was done in Institutional Department of Oral Medicine and Radiology for 140 patients, out of which 100 patients consented to participate in our study.
The patients were randomly allocated equally using a computerized slip generation procedure (ratio of 1:1) in Microsoft Excel, 2021 using INDEX, SORTBY, and SEQUENCE Functions for Mac Version 16.16.10. irrespective of age and sex. A punch biopsy of the lesion was obtained 1 week prior to excision. The two treatment groups in the present study were based on the dressing materials/grafts used:
Group 1: Excised lesions were grafted with PRF membrane.
Group 2: Excised lesions were grafted with collagen membrane
Inclusion criteria
Healthy ASA class − I patients in the age group of 18–60 yr.
Patients with superficial mucosal lesions (Those lesions which on clinical palpation do not appear to be fixed or invaded into the underlying connective tissue and punch biopsy of the lesion reveal involvement of only the mucosal epithelium with absence of severe dysplasia).
Sufficiently large lesions in which primary closure of post-excision surgical defect is not possible.
Patients who developed the white lesions secondary to the habit of consumption of tobacco and related products.
Exclusion criteria
Patients with systemic diseases, ASA Grade II to VI.
Patients undergoing chemotherapy or radiotherapy.
Patients on long-term antibiotic, steroid or antiplatelet drugs.
Active local and/or systemic infection.
Pregnant and lactating women and those taking oral contraceptives.
Surgical overview
A detailed case assessment was done for every patient enrolled in our study with history recording, alongside relevant blood parameters and clinical photographs. Hemostatic biological constants were set at Hemoglobin level greater than 9 g/dL, and the platelet count greater than 100 × 109/L. Patient were asked to rinse with 0.12% chlorhexidine gluconate mouthwash prior to procedure, and extraoral skin preparation were done using 5% betadine solution, with sterile draping. All the surgical procedures were performed by the same surgeon under local anesthesia 2% lignocaine with 1:80,000 adrenaline. In each case, wide scalpel excision of the lesion was performed with a safe normal mucosal margin of 5 mm, creating a partial thickness wound (defined by excision up to the submucosal depths only and not involving the underlying muscle).
For Group 1, PRF membrane was fabricated and grafted to the wound. The protocol for fabrication of the PRF clot and membrane was followed as:
The required quantity of blood (10 mL) was drawn from the patient's peripheral vein and transferred into sterile calibrated glass test tube (without anticoagulants) and immediately centrifuged at 3,000 rpm for 10 min in a laboratory centrifuge machine (REMI, R-8C). A 3-layered structure was obtained, either immediately or after thawing the fluid for a few minutes after centrifugation. A structured PRF clot formed in the middle of the tube, below which was the red blood cells, with the topmost layer, a thin layer of supernatant plasma (platelet poor plasma [PPP]). The PRF clot was drawn with the help of a tweezers and pressed between 2 moist gauze-covered glass slabs of standard size for 30 seconds to obtain a membrane of adequate thickness of around 0.6 mm. Until use, the membrane was kept covered in the wet gauze and humidified routinely with the supernatant plasma (PPP) that had remained in the glass tube and saline. In cases where additional PRF membranes were required for complete coverage of the defect, more autologous blood was taken as per requirement.
For Group 2, an alloplastic sterile bovine collagen membrane was used (DR. THACHARODI'S XENODERM WT/WTM, manufactured by Helix Pharma, No.15/16, Cuddalore Road, Tollgate, Ariankuppam, Puducherry- 605007, India). The collagen used in this study was thin acellular re-constituting translucent sheet made up of bovine type-1 collagen of 5 × 5 cm2 dimension and thickness around 0.6 mm which was sufficient to cover the entire buccal mucosa. Unlike conventional wet sheet, it was a reconstituting freeze-dried sheet without alcohol preservative. Collagen membrane was soaked twice in normal saline for minimum 5 min for imbibing water and to remove the preservative solution from its surface. This made the membrane soft and supple and it mimicked the native skin graft. The membrane was adapted to the mucosal defect and trimmed to appropriate size with scissors.
Quilt suturing using vicryl sutures (3-0) were done for fixing both the membrane in the centre and in the periphery, to handle the wound drainage. After final suturing was completed, a Vaseline-chlorhexidine impregnated gauze was used for pressure dressing and it was removed after 24 h. The descriptive patient photos are from Figures 1–8.
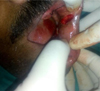 |
Fig. 1 Excision of the lesion. |
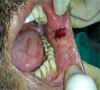 |
Fig. 2 PRF membrane–quilt suturing. |
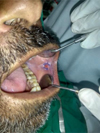 |
Fig. 3 7 days post–operatively. |
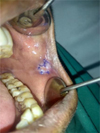 |
Fig. 4 30 days post–operatively. |
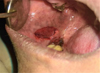 |
Fig. 5 Excision of the lesion. |
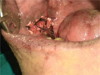 |
Fig. 6 Collagen membrane–quilt suturing. |
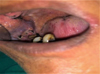 |
Fig. 7 7 days post–operatively. |
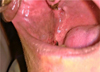 |
Fig. 8 30 days post–operatively. |
Post-operative care
All the patients were advised soft diet for 7 days and were also instructed to maintain strict oral hygiene. Postoperatively, tablet aceclofenac SOS was prescribed to the patients. All the excised specimens were sent for histopathological analysis. The patients were followed up for one month with recall visit at 3rd day, 7th day and 30th day.
Outcome parameters to be evaluated
Conformability of both PRF and Collagen Membrane were checked prior to grafting for every patient. Hemostasis in operated site was achieved in all cases of both study group, when checked at 1st hour. Adherence of the membranes were present for both groups as checked at 7th post-operative day.
Post-operative day
Pain: Pain was evaluated using a numerical Visual Analog Scale (VAS), having markings from a minimum score 0 to a maximum score 10 at 3rd and 7th day respectively.
-
Clinical Healing: Redness, Edema and Suppuration were individually assessed at 7th and 30th day:
Clinical Healing Score (Sum of 3 Criteria)- The sum of these 3 criteria is the clinical healing score; the closer the score is to 0, the better the healing, and vice versa.
Criteria Score Redness Absent − 0 Redness Present − 1 Edema Absent − 0 Edema Present − 1 Suppuration Absent − 0 Suppuration Present − 1 -
Granulation Tissue: The presence of granulation tissues was noted at the end of 1st week, checked in accordance to scoring pattern of Bessho and Murakami [23] as:
2-Good (entire wound).
1-Fair (nearly the entire wound).
0-Poor (inadequate).
-
Epithelialization: Epithelialization was checked in accordance to scoring pattern of Bessho and Murakami [15] at 30th day as:
2-Good (entire wound).
1-Fair (nearly the entire wound).
0-Poor (inadequate).
-
Wound Contracture: Contracture of the wound site was usually measured by the amount of mouth opening preoperatively and postoperatively. This was assessed in accordance to scoring pattern of Bessho and Murakami [15] at 30th day as:
2-Good (none/ <25%).
1-Fair (<50%)
0-Poor (severe/ >50%).
-
Complications: Scar hypertrophy, Fibrosis, Recurrence, Vestibular depth reduction and any others (if any) were individually assessed at 7th and 30th day:
Absent-score 0.
Present- score 1.
-
Statistical analysis
Data was analyzed using the statistical package SPSS 22.0 (SPSS Inc., Chicago, IL) and level of significance was set at p < 0.05. Descriptive statistics was performed to assess the mean and standard deviation of the respective groups. Normality of the data was assessed using SHAPIRO WILKINSON TEST. Inferential statistics to find out the difference between the groups was done using T TEST.
Results
The present study comprised of 100 patients (54 females and 46 males) with the demographic details for both the groups is enlisted in Table I. The difference in both groups for demographic comparison was not statistically significant. Out of the 50 patients in Group 1, 23 patients consumed gutkha, 10 patients consumed kharra, 13 patients with tobacco chewing habits and 4 patients with multiple adverse habits. Similarly in Group 2, 26 patients consumed gutkha, 11 patients with habits of kharra, 10 patients with tobacco chewing habits and remaining 3 patients with multiple adverse habits. The statistical differences for these adverse habits amongst both the groups were found to be insignificant (p > 0.5). The average duration of the habits when assessed for patients in both the groups was found to be in the average duration of 5 yr to 15 yr, which was statistically not significant (p > 0.5). The site of the lesion in our study were mostly in right and/or left buccal mucosa, near the commissural region of lips, the lower labial mucosa and the adjacent vestibule for both Group 1 and Group 2. The distribution of different potentially malignant oral lesions can be summarized as 46 cases of Leukoplakia, 30 cases of Tobacco Pouch Keratosis, 12 cases of Oral Submucous Fibrosis (OSMF), 8 cases of Lichen planus and 4 cases of Erythroplakia for a total of 100 patients enrolled for the present study. The statistical difference for these lesions were observed as not significant (p > 0.5), when assessed for both the groups, and is summarized in Table II. A general statistical distribution of all variables has been listed in Table III.
Demographic details.
Summary of patient habits with associated lesions.
On comparing post-operative pain among the study groups, better reduction in pain was observed in group 1 compared to group 2 with statistically significant difference (p<0.05) amongst 3rd and 7th day (Tab. III).
On comparing clinical healing between the study groups, better clinical healing was observed in group 1 compared to group 2 with statistically significant difference (p<0.05), between 7th day and 30th day (Tab. III).
The comparison for presence of granulation tissue in between study groups at 7th day reported significant difference (p<0.05), where group 1 reported a good granulation presence compared to group 2 (Tab. III).
On the comparison of Epithelialization and contracture of wounds among the study groups. the analysis reported no significant difference (p>0.05), as depicted in Table III.
Complications at the end of follow-up period were seen in 3 patients amongst the groups, which were well managed conservatively. These findings were statistically insignificant (p > 0.05), as depicted in Table III.
General statistical distribution of all variables.
Discussion
The excision of potentially malignant oral lesions has been the preferred treatment modality compared to conservative medical management. Van der waal suggested to excise or lase any oral or oropharyngeal leukoplakia or erythroplakia, irrespective of the presence or absence of dysplasia [7]. Thomas et al. [24] performed cold knife surgical excision of oral leukoplakia in 70 patients, of whom 68.6% remained disease free, with no evidence of recurrence or new lesions. Vedtofte et al. [25] evaluated the use of cryosurgery and laser surgery for the treatment of oral premalignant lesions and reported that a major disadvantage in their use is that the whole lesion is not available for histologic examination. Hence, we chose scalpel excision of potentially malignant lesions, followed by the use of a dressing material for wound coverage.
The primary purpose of a coverage agent is to protect the wound and provide an environment conducive to healing. Four classes of dressing are available for selection at present. These including the open dressing, such as fine-mesh gauze (non-adherent), semi-open dressings (highly water permeable), occlusive dressings (occlusive to the passage of moisture and other substance), and Synthetic Adhesive Moisture (SAM − vapor permeable) [26]. Autologous grafts [7], alloplastic grafts [8], Local flaps (i.e., mucosal flaps, tongue flap, nasolabial flap, palatal island flaps), buccal fat pad, and distant flaps have a risk of donor site morbidity, allergic reactions with the added cost of surgery and difficulty in procurement [27].
The gold standard for in vivo tissue healing and regeneration requires the mutual interaction between a scaffold (fibrin matrix), platelets, growth factors, leukocytes, and stem cells [28]. These key elements are active components of Autologous PRF and Collagen Membrane, both of which are involved in the key processes of tissue healing and regeneration, and are widely used in oral surgery, plastic surgery, craniofacial surgery, cardiac surgery, orthopedics, neurology, sports medicine, and dermatology [29].
Autologous platelet rich fibrin (PRF) membrane was used by Mohanty S, et al. [30] and Pathak H, et al. [5] as dressing materials for soft tissue defects after excision of oral mucosal lesions. When platelets in PRF are activated by thrombin, thrombocytes release high quantities of three main growth factors [31], which are: transforming growth factor b-1 (TGF beta-1), platelet-derived growth factor AB (PDGF-AB), vascular endothelial growth factor (VEGF), and an important coagulation matrixcellular glycoprotein (thrombospondin-1, TSP-1) during 7 days, that serve to accelerate wound healing by increasing cell proliferation and differentiation, extracellular matrix synthesis, osteoid production, chemotaxis, angiogenesis (neo-vascularization), and collagen synthesis [32]. Platelets also store other bioactive proteins, such as the enzyme matrix metalloproteinase (MMP), coagulation factors, chemotactic factors, adhesion molecules, vasoactive substances, as well as some bactericidal and fungicidal proteins that also play an active role in the wound healing process [33]. PRF polymerize to form a three‑dimensional structure with platelet cytokines entrapped in fibrin mesh, ensures slow and continuous release of growth factors over time [34,35] which has shown to be advantageous for the bone graft healing process [36] and angiogenesis [37].
A study conducted by Azar Dt et al. [38] and Thorat M et al. [39] showed that vital fibrin could be used as a membrane to cover bone augmentation sites. PRF is effective, in particular in the first phases of wound healing, and its effectiveness may change depending on the characteristics of jointly applied graft material [40–41].
Clinically, the PRF membrane used in our study was thin but had sufficient strength to resist tearing when handled carefully. Care was taken to use it as early as possible after making the membrane, otherwise exposure to the atmosphere makes the membrane dry and eventually leads to shrinkage and loss of its structural integrity, as reported in a study by Pathak H, et al., (2015) [5]. The membrane had a good texture and suppleness, hence was easily adapted to the tissue of any contour without distorting its integrity. The thickness of the membrane was not less than 0.5 mm, as suggested by Ehrenfest D, et al. (2010) [10]. The PRF eventually appears to resorb or dissolve within the tissue as the healing and surface epithelialization begins [5]. The thickness of the PRF epithelium increases significantly beginning on day three, indicating that epithelialization in the PRF membrane group occurs rapidly, when used as surgical dressing for open wound coverage.
Collagen membrane has been a preferred choice in oral and maxillofacial surgical procedures as a graft or a dressing material, owing to its inherent property of haemostatic effect as it is a specific activator of platelets and helps in their adhesion to collagen fibre, aggregation and thus strengthens the clot [42]. Collagen being a natural substrate of extracellular matrix, it is chemotactic to various cell types such as endothelial cells, fibroblasts there by leading to reduced inflammatory process which contributes to reduced pain and burning sensation during healing, which is in accordance with the studies by Raghavendra Reddy et al. [43] Shobha Natraj et al. [9] Shanmugam et al. [44] and Rastogi et al. [45].
The wound healing by collagen may be explained through the formation of a gelatinized coagulum containing abundant amount of fibrinogen and fibronectin which contains high concentrations of chemo attractants, growth factors help in deposition and organization of freshly formed fibres [46,47]. The collagen-based dressing reduce the inflammatory response, upregulates growth factor levels, like TGF-β, VEGF and bFGF, promotes collagen deposition, ECM synthesis and granulation tissue thickness, and recruits fibroblasts to enhance cell viability and proliferation to accelerate wound healing [9,18,18,48].
The collagen used in this study was re-constituting translucent sheet, which refers that collagen is cross-linked with tanning agents such as glutaraldehyde or chromium sulphate so that its tensile strength is improved, and it becomes insoluble, its resorption rate is slowed down, and its antigenicity is markedly lowered. It was supple and adapted well to the wound irrespective of its contour, adheres well to the tissues. The membrane was stable and strong enough to resist the masticatory forces when properly adapted and sutured, though suturing of collagen was time consuming [49].
Both PRF and collagen membrane are widely used in the reconstructive procedures and share few similar properties, but differ in their basic nature as a graft. There have been many studies which have individually studied the healing of intraoral raw wounds using either PRF [6,19] or collagen membrane [8,9,20]. But, to our knowledge, there has been no detailed study which has compared the healing efficacy of PRF and collagen membrane in intraoral soft tissue defects. Thus, this study aims to compare and evaluate the healing pattern after surgical excision of oral mucosal lesions by using PRF membrane and collagen membrane.
Pain was assessed at 3rd and 7th day follow-up and better pain reduction was observed in PRF Group. The results are in accordance with the studies by Kumar et al. [50] Bilginaylor et al. [51] Uyanik LO et al. [52] Al-Hamed et al. [53] Kulkarni et al. [54] and Mahajan M et al. [55] who also observed pain decreases significantly at 1st, 3rd and 7th day following PRF usage. However, a difference in results was observed in study by Mohamad El Masri., et al. [56] which showed insignificant effect of PRF on pain perception in comparison with the effect of collagen membrane, which they attributed to different surgical sites.
Clinical Healing was found accelerated in PRF Group at 7th day. During the 30th day follow-up all patients in both the groups presented with complete clinical healing. These findings signify that complete healing of the soft tissue defect will take place within 30 days, and is accelerated when PRF is used, and is in accordance with the studies by Mahajan M et al. [57] and Singh et al. [57] In another study by Ehrenfest DM et al. [58] and Desai CB et al. [59] faster healing was observed in PRF group initially till 12th postoperative day. A study by C Wang et al. [60] comparing the healing of the soft tissue defects, showed that PRF, Collagen membrane and the Control group were completely healed at the 14th day, 21st day and 28th day respectively after surgery, and the wound tissue was finally healed. However, a study by Shanmugam M. et al. [58] showed comparative acceleration in wound healing with greater firmness in the tissues 2 weeks postoperatively with collagen sponge application.
It was found that the PRF and collagen membrane showed partial resorption by the 7th postoperative day and complete resorption by the 30th postoperative day in all patients, as they exhibit fibrin collagen interaction resulting in the initial adherence to the underlying tissues, which is in accordance with the study by ES Gorell et al. [61] Healing Parameters such as presence of Granulation tissue, Wound Epithelialization, and Contracture of wounds were assessed at 30th day follow-up, showing insignificant differences in between the groups, with PRF Group proving better than Collagen Group.
The findings for complications at the end of follow-up period were statistically insignificant in the present study. Mild discomfort and mild to moderate pain at the site of surgery was reported by 3 patients amongst both the groups. Such patients were reassured, kept under observation and advised massage using 0.1% triamcinolone acetonide ointment twice daily. None of the patients from both groups had recurrence of lesion as observed. This is in accordance with the findings of Reddy et al. [47] Shoba Natraj et al. [9] substantiating that PRF and Collagen membrane are safe biological dressing materials.
Within the scope of the study, we found both PRF and Collagen to be excellent dressing materials, with PRF outperforming Collagen in the initial stages of healing and its multifaceted benefits proves its mettle as a better dressing material when used after surgical excision of superficial, potentially malignant lesions of oral cavity.
Conclusion
The clinical observations along with statistical analysis for comparison of healing between two groups with post-operative parameters (Pain, Clinical Healing and Granulation Presence) indicate significantly better results with PRF, with better reduction in pain, improved clinical healing and good granulation presence as compared to collagen group. Although, the post-operative parameters checked at 30th day (Epithelialization, Wound Contracture, and Complications) showed no significant differences in between the two groups.
Practical implications
PRF (Platelet Rich fibrin) is an autologous derived membrane with beneficial actions on clinical wound healing as well as it proves its mettle as a better dressing material when used after surgical excision of superficial, potentially malignant lesions of oral cavity.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This research did not receive any specific funding.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee (SDDHDC/IEC/2021/06) and with the 1964 Declaration of Helsinki and its later amendments of 2013 or comparable ethical standards.
Informed consent
All the surgical and experimental procedures were explained verbally and in writing, and informed written informed consent was obtained from all patients and/or families before enrolment.
Authors contributions
Dr. Vishal Kumar Poddar: Conceptualization, Methodology, Writing original draft. Dr. Srimathy S. Arora: Visualization, Investigation, Supervision. Dr. Kusum Kumari: Writing- Reviewing and Editing.
Acknowledgements
The authors extend their gratitude towards Dr Abdul Saheer, HOD, Department of Public Health Dentistry, Al Azhar Dental College, Kerela for constant help on statistics and experimental design with proper interpretations.
References
- Mohammed A, et al. Clinicopathologic correlation of white, non-scrapable oral mucosal surface lesions: a study of 100 cases. J Clin Diagn Res 2016;10:ZC38–ZC41. [Google Scholar]
- Naveen-Kumar B, Tatapudi R, et al. Various forms of tobacco usage and its associated oral mucosal lesions. J Clin Exp Dent 2016;8:e172–e177. [PubMed] [Google Scholar]
- Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 2007;36:575–580. [CrossRef] [PubMed] [Google Scholar]
- Behura SS, Masthan MK, Narayanaswamy AB. Oral mucosal lesions associated with smokers and chewers − a case-control study in chennai population. J Clin Diagn Res 2015;9:ZC17–ZC22. [Google Scholar]
- Pathak H, Mohanty S, Aadithya B, Dabas J. Treatment of oral mucosal lesions by scalpel excision and platelet-rich fibrin membrane grafting: a review of 26 sites. J Oral Maxillofac Surg 2015;73:1865–1874. [CrossRef] [PubMed] [Google Scholar]
- Shanmugam D, Dominic N. Evaluation of bovine-derived collagen membrane in oral surgical mucosal defects. J Maxillofac Oral Surg 2018;1172–1176. [Google Scholar]
- Rahman A, Packiaraj I, et al. Role of collagen membrane- a comprehensive review. J Adv Med Dent Sci Res 2015;3:95–97. [Google Scholar]
- Poornima Sowjanya N, Rao NM, et al. Versitality of the use of collagen membrane in oral cavity. J Clin Diagn Res 2016;10:ZC30–ZC33. [PubMed] [Google Scholar]
- Natraj S, Guruprasad Y, Jaya Shetty PN. A comparative clinical evaluation of buccal fat pad and collagen in surgical management of OSMF. Arch Dent Sci 2011;2:17–24. [Google Scholar]
- Ehrenfest DM, Del Corso M, et al. Three‑dimensional architecture and cell composition of a Choukroun's platelet‑rich fibrin clot and membrane. J Periodontol 2010;81:546–555. [CrossRef] [PubMed] [Google Scholar]
- Sunitha R, Munirathnam N. Platelet-rich fibrin: evolution of second generation platelet concentrate. Indian J Dent Res 2008;19:42. [CrossRef] [PubMed] [Google Scholar]
- Dohan DM, Choukroun J, Diss A, et al. Platelet‑rich fibrin (PRF): a second‑generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e37–e44. [CrossRef] [PubMed] [Google Scholar]
- Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second generation platelet concentrate. Part III: Leukocytes activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:E51. [Google Scholar]
- Reibel J, Gale N, Hille J, et al. Oral potentially malignant disorders and oral epithelial dysplasia. In: El-Naggar AK, Chan JKC, Grand JR, Takata T, Slootweg PPJ, Eds. WHO Classification of Head and Neck Tumours. 4th ed. Lyon, France: IARC 2017, p. 112–5. [Google Scholar]
- Bessho K, Murakami K, Iizuka T. The use of a new bilayer artificial dermis for vestibular extension. Br J Oral Maxillofac Surg 1998;36:457. [CrossRef] [PubMed] [Google Scholar]
- Mohamad El Masri, et al. Comparison of PRF versus collagen membrane on healing of socket and bone formation (Randomized Split Mouth Design). Acta Sci Dent Sci 2018;2.7:13–22. [Google Scholar]
- Wang C, Ma X. Preliminary study on the healing effect of PRF for soft tissue defects in oral implants. J King Saud Univ Sci, 2020;82:21–26 [Google Scholar]
- Sun L, et al. A collagen-based bi-layered composite dressing for accelerated wound healing. J Tissue Viability, 2022;31:180–189. [Google Scholar]
- Mohanty S, Pathak H, Dabas J. Platelet rich fibrin: a new covering material for oral mucosal defects. J Oral Bio Craniofac Res 2014;4:144–146. [CrossRef] [Google Scholar]
- Rastogi S, Modi M, Sathian B. The efficacy of collagen membrane as a biodegradable wound dressing material for surgical defects of oral mucosa: a prospective study. J Oral Maxillofac Surg 2009;67:1600–1606. [CrossRef] [PubMed] [Google Scholar]
- Van der Waal I. Oral Leukoplakia; a proposal for simplification and consistency of the clinical classification and terminology. Med Oral Patol Oral Cir Bucal 2019;24:e799. [PubMed] [Google Scholar]
- Singh A, Kohli M, Gupta N. Platelet rich fibrin: a novel approach for osseous regeneration. J Maxillofac Oral Surg 2012;11:430–434. [CrossRef] [PubMed] [Google Scholar]
- Warnakulasuriya S, Kujan O, et al. Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, Convened by the WHO Collaborating Centre for Oral Cancer. Oral Diseases, 2020. [Google Scholar]
- Thomas G, Kunnambath R, et al. Long term outcome of surgical excision of leukoplakia in a screening intervention trial. J Indian Acad Oral Med Radiol 2012;24:126. [CrossRef] [Google Scholar]
- Vedtofte P, Holmstrup P, Hjørting-Hansen E, et al. Surgical treatment of premalignant lesions of the oral mucosa. Int J Oral Maxillofac Surg 1987;16:656. [CrossRef] [PubMed] [Google Scholar]
- El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours (IARC WHO classification of tumours). 4th edn. World Health Organization, 2017. [Google Scholar]
- Farah CS, Woo SB, Zain RB, et al. Oral cancer and oral potentially malignant disorders. Int J Dent 2014;853479. [PubMed] [Google Scholar]
- Huang JJ, Wallace C, Lin JY, et al. Two small flaps from one anterolateral thigh donor site for bilateral buccal mucosa reconstruction after release of submucous fibrosis and/or contracture. J Plast Reconstr Aesthet Surg 2010;63:440. [CrossRef] [PubMed] [Google Scholar]
- Kawase T, Kamiya M, et al. The heat-compression technique for the conversion of platelet-rich fibr in preparation to a barrier membrane with a reduced rate of biodegradation. J Biomed Mater Res. Part B, Appl Biomater 2015;103:825–831. [CrossRef] [Google Scholar]
- Sun X-L, Zhou Y-M, et al. The effect of platelet rich fibrin on biologic characteristics of osteoblasts. Shanghai Kou Qiang Yi Xue Shanghai J Stomatol 2015;24:61–64. [Google Scholar]
- Reddy SS, Prashanth R, Yashodha Devi BK, et al. Prevalence of oral mucosal lesions among chewing tobacco users: a cross-sectional study. Indian J Dent Res 2015;26:537–541. [CrossRef] [PubMed] [Google Scholar]
- Jayadev M, Marshal Vr, Karunakar N. Role of Platelet-rich fibrin in wound healing: a critical review. J Conserv Dent 2013;16:284. [CrossRef] [PubMed] [Google Scholar]
- Azar DT. Refractive surgery 3rd ed St. Louis, Elsevier Health Sciences, 2019, p. 238. [Google Scholar]
- Thorat M, Pradeep AR, Pallavi B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: a controlled clinical trial. J Clin Periodontol 2011;38:925–932. [CrossRef] [PubMed] [Google Scholar]
- Chang YC, Zhao JH. Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust Dent J 2011;56:365–371. [CrossRef] [PubMed] [Google Scholar]
- Saman W. Clinical features and presentation of oral potentially malignant disorders. Oral Surg Oral Med Oral Pathol Oral Radiol 2018;125:582–590. [CrossRef] [PubMed] [Google Scholar]
- Vijayalakshmi R, Rajmohan CS, Deepalakshmi D, Sivakami G. Use of platelet rich fibrin in a fenestration defect around an implant. J Indian Soc Periodontol. 2012;16:108–112. [CrossRef] [PubMed] [Google Scholar]
- Reiter D. Methods and materials for wound management. Otolaryngol Head Neck Surg 1994;110:550–556. [CrossRef] [PubMed] [Google Scholar]
- Kawase T. Platelet-rich plasma and its derivatives as promising bioactive materials for regenerative medicine: basic principles and concepts underlying recent advances. Odontology 2015;103.2:126–135. [CrossRef] [PubMed] [Google Scholar]
- M Dohan Ehrenfest D, et al. In search of a consensus terminology in the field of platelet concentrates for surgical use: platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin gel polymerization and leukocytes. Curr Pharm Biotechnol 2012;13.7:1131–1137. [CrossRef] [PubMed] [Google Scholar]
- Del Corso M, Toffl er M, Ehrenfest DM, et al. Use of an autologous leukocyte and platelet-rich fibrin (L-PRF) membrane in post-avulsion sites: an overview of Choukroun's PRF. J Implant Adv Clin Dent 2010;1:27–35. [Google Scholar]
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006b;101:e45e–e50. [CrossRef] [PubMed] [Google Scholar]
- Chignon‑Sicard B, Georgiou CA, et al. Efficacy of leukocyte‑and platelet‑rich fibrin in wound healing: a randomized controlled clinical trial. Plast Reconstr Surg 2012;130:819–829. [Google Scholar]
- Buser D, Chappuis V, Belser UC, Chen S. Implant placement post extraction in esthetic single tooth sites: when immediate, when early, when late? Periodontol 2000 2017;73:84–102. [CrossRef] [PubMed] [Google Scholar]
- Warnakulasuriya S, Ariyawardena A. Malignant transformation of oral leukoplakia − a systematic review. J Oral Pathol Med 2016;45:155–156. [CrossRef] [PubMed] [Google Scholar]
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc 2014;145:45–56. [CrossRef] [PubMed] [Google Scholar]
- Raghavendra Reddy Y, Srinath N, Nandakumar H, Rajini Kanth M. Role of collagen impregnated with dexamethasone and placentrix in patients with oral submucous fibrosis. J Maxillofac Oral Surg 2012;11:166–170. [CrossRef] [PubMed] [Google Scholar]
- Singh O, Gupta SS, Mosses S, Shukla S. Collagen dressing versus conventional dressings. J Cutan Aesthet Surg 2011;4:12–16. [CrossRef] [PubMed] [Google Scholar]
- Thoma DS, Sancho-Puchades M, Ettlin DA, Hämmerle CHF, Jung RE. Impact of collagen matrix on early healing, aesthetics and patient morbidity in oral mucosal wounds − a randomized study in humans. J Clin Periodontol 2012;39:157–165. [CrossRef] [PubMed] [Google Scholar]
- Charulatha V, Rajaram A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials 2003;24:759–767. [CrossRef] [PubMed] [Google Scholar]
- Manon‐Jensen T, Kjeld NG, Karsdal MA. Collagen‐mediated hemostasis. J Thromb Haemost 2016;14:438–448. [CrossRef] [PubMed] [Google Scholar]
- Kumar N, et al. Evaluation of treatment outcome after impacted mandibular third molar surgery with the use of autologous platelet-rich fibrin: a randomized controlled clinical study. J Oral Maxillofac Surg 2015;73.6:1042–1049. [CrossRef] [PubMed] [Google Scholar]
- Bilginaylar K, Uyanik LO. Evaluation of the effects of platelet- rich fibrin and piezosurgery on outcomes after removal of impacted mandibular third molars. Brit J Oral Maxillofac Surg 2016;54.6:629–633. [CrossRef] [Google Scholar]
- Uyanık LO, et al. Effects of platelet-rich fibrin and piezosurgery on impacted mandibular third molar surgery outcomes. Head Face Med 2015;11:25–32. [CrossRef] [PubMed] [Google Scholar]
- Al-Hamed FS, et al. Clinical effects of platelet-rich fibrin (PRF) following surgical extraction of lower third molar. Saudi J Dent Res 2017;8.1-2:19–25. [CrossRef] [Google Scholar]
- Kulkarni MR, Thomas BS, Varghese JM, Bhat GS. Platelet‑rich fibrin as an adjunct to palatal wound healing after harvesting a free gingival graft: a case series. J Indian Soc Periodontol 2014;18:399–402. [CrossRef] [PubMed] [Google Scholar]
- Mahajan M, Gupta Mk, Bande C, Meshram V. Comparative evaluation of healing pattern after surgical excision of oral mucosal lesions by using platelet rich fibrin (prf) membrane and collagen membrane as grafting materials- a randomized clinical trial. J Oral Maxillofac Surg 2018;S0278-2391:30195–2. [Google Scholar]
- Shanmugam M, Kumar TS, Arun KV, Arun R, Karthik SJ. Clinical and histological evaluation of two dressing materials in the healing of palatal wounds. J Indian Soc Periodontol 2010;14:241–244. [CrossRef] [PubMed] [Google Scholar]
- Gorell ES, Leung TH, Khuu P, Lane AT. Purified type I collagen wound matrix improves chronic wound healing in patients with recessive dystrophic epidermolysis bullosa. Pediatr Dermatol 2015;32:220–225 [PubMed] [Google Scholar]
- Dohan Ehrenfest DM, Diss A, Odin G, et al. In vitro effects of Choukroun's PRF (platelet‑rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:341–352. [CrossRef] [PubMed] [Google Scholar]
- Desai CB, Mahindra UR, Kini YK, Bakshi MK. Use of platelet‑rich fibrin over skin wounds: modified secondary intention healing. J Cutan Aesthet Surg 2013;6:35–37. [CrossRef] [PubMed] [Google Scholar]
All Tables
All Figures
 |
Fig. 1 Excision of the lesion. |
| In the text | |
 |
Fig. 2 PRF membrane–quilt suturing. |
| In the text | |
 |
Fig. 3 7 days post–operatively. |
| In the text | |
 |
Fig. 4 30 days post–operatively. |
| In the text | |
 |
Fig. 5 Excision of the lesion. |
| In the text | |
 |
Fig. 6 Collagen membrane–quilt suturing. |
| In the text | |
 |
Fig. 7 7 days post–operatively. |
| In the text | |
 |
Fig. 8 30 days post–operatively. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


