| Issue |
J Oral Med Oral Surg
Volume 26, Number 1, 2020
|
|
|---|---|---|
| Article Number | 9 | |
| Number of page(s) | 5 | |
| Section | Cas clinique et revue de la littérature / Up-to date review and case report | |
| DOI | https://doi.org/10.1051/mbcb/2019034 | |
| Published online | 03 February 2020 | |
Up-to Date Review And Case Report
Ectopic teeth in a patient with Gorham-Stout disease previously treated by biphosphonates: a case report
1
Department of Cranio-Maxillo-Facial Surgery of the Pediatric Hospital, Hôpital Femme Mère Enfant, 59 Boulevard Pinel, 69677 Bron, France
2
Faculté d'Odontologie, Université Claude Bernard Lyon 1, Rue Guillaume Paradin, 69008 Lyon, France
* Correspondence: melanie.le-donne@hotmail.fr
Received:
16
April
2019
Accepted:
21
October
2019
Introduction: Gorham-Stout disease is a rare idiopathic condition of the bone that is characterized by a massive and spontaneous osteolysis, with a vascular or lymphatic proliferation in bone, which is then replaced by fibrous tissue. Observation: a 16-year-old patient was referred to the maxillofacial surgery department to remove ectopic teeth bilaterally in the ramus. He had Gorham-Stout disease, managed for many years in orthopedic surgery department for a lower limb lesion and in neurosurgery department for a breach of the meninges. He was treated for 4 years with bisphosphonates. The removal of the ectopic teeth went well, with a simple postoperative course. Discussion: Gorham-Stout disease physiopathology remains unknown. Facial bones are often involved, especially the mandible. There are many possible treatments, but, due to the rarity of the disease, no therapeutic consensus exists. Bisphosphonates seem to be a good way to control this condition. So far, no case of bisphosphonates related osteonecrosis of the jaw has been reported in children. Conclusion: Gorham-Stout disease can involve the mandible and may lead to ectopic teeth.
Key words: Gorham-Stout disease / ectopic teeth / rare disease / biphosphonates
© The authors, 2020
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Gorham–Stout disease (GSD), also known as Gorham's disease or vanishing bone disease, is a rare osseous condition that is characterized by massive, spontaneous osteolysis, with blood or lymphatic vessel proliferation in the bone, which is subsequently replaced by fibrous tissue [1–5].
GSD involves the idiopathic and progressive osteolysis of one or more contiguous bones surrounding an initial site, regardless of the sutures. Although this disease can affect any skeletal bone, it is most frequently reported in the skull, shoulders, and pelvic girdle [2].
Observation
A 16-year-old male with GSD was referred to the department of maxillofacial surgery for the management of mandibular retrognathia that was causing sleep apnea. The patient had been monitored for several years for lesions on the iliac crest and right femur (Fig. 1) that had resulted in numerous fractures of the lower limbs (femoral diaphysis fracture of the middle one-third and proximal one-third in 2008); he had been treated by neurosurgeons for repeated episodes of meningitis affecting the ethmoidal osteomeningeal breccia along with chronic rhinorrhea, which contraindicated nasotracheal intubation.
For 4 years (between the ages of 5 and 9 years), the patient received treatment with pamidronate (cumulated dose of 564 mg over 2 years and a half) followed by zolendronic acid (cumulated dose of 5,1 mg over 6 months); further, he underwent interferon α treatments for the lower limbs, 7 years before the oral surgery consultation.
Due to several bouts of meningitis, the patient was receiving long term Oracillin antibiotic therapy (2 000 000 UI/day). From the maxillofacial perspective, a severe malocclusion associated with marked mandibular retrognathia and dental ectopia was observed. The orthopantomogram showed teeth 48 and 38 at the coronal level and 37 and 47 in the rising branch − all four were noted proximal to the lower alveolar nerves, whose function was otherwise normal. A thinning of the mandible, presence of dental apices in the basilar bone, and loss of lamina dura over the entire mandible were observed. The alveolar bone appeared to exhibit slight mineralization, and condyles were extremely thin (Fig. 2).
Using facial teleradiology, some bone damage was detected over the entire calvaria (Fig. 3).
According to the orthodontic consultation, it was decided to remove the ectopic teeth and persistent temporary teeth. The patient and his patients were informed regarding the surgical risks involved (mandibular fracture risk and nerve risk), and his parents provided their consent for the procedure.
Under general anesthesia with oral intubation, an incision was made behind the vestibule facing the teeth upward on the rising branch; this was followed by the detachment of a mucoperiosteal flap. A slight lingual clearance was used to allow better visibility of the teeth by protecting the lingual nerve. To avoid any fracture risk, a bone window was prepared facing the teeth before their careful extraction. At the end of the procedure, the lower alveolar nerve was visible at the bottom of the socket and was preserved. The operation was successful, and no postoperative complications were observed (Figs. 4 and 5).
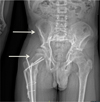 |
Fig. 1 Pelvis radiograph showing the bone involvement of the Gorham Stout Disease at the right iliac crest and femur (arrows). |
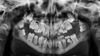 |
Fig. 2 Pre operative orthopantomogram. |
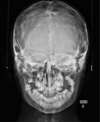 |
Fig. 3 Frontal teleradiograph. |
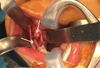 |
Fig. 4 Intra oral intraoperative photograph showing 47 48 position (arrow). |
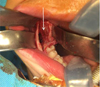 |
Fig. 5 Intra oral intraoperative photograph showing inferior alveolar nerve at the bottom of the teeth sockets (arrow). |
Discussion
GSD is a rare condition, with approximately 200 cases reported in the literature [2,3]. Moreover, the exact pathophysiological etiology remains unknown. It appears as a sporadic disease caused by specific genetic risk factors or by mosaicism for another somatic mutation [4].
The lymphatic vessels, not the blood vessels as originally suspected, appeared to be initially affected. Uncontrolled growth of the lymphatic vessels could result in osteolysis by bone compression. Blood vessel growth factors, such as vascular endothelial growth factor (VEGF), would play a central role in this anarchic development. The role of osteoclasts is discussed, as they are not highlighted in all lesions [2,4,6].
Clinically, there is no correlation with sex or age, although most cases of GSD are identified before the age of 40 years. The pelvis, shoulders, and skull are the most frequently affected areas [2–4].
Clinical manifestations depend on the affected site − the disease manifestations can include sudden swelling and pain of pathological fracture for most of the localization, or it can provoke chronic pain and progressive limitation of movement when limbs are affected [2].
Facial bone damage is reported in 30% of the cases of GSD [7]. The mandible is the most frequently affected bone, with 60 cases reported in the literature. The main symptom is intermittent pain and swelling [2,6,8,9]. The teeth in the affected area become loose and eventually fall out [2,6,9,10]. Functional abnormalities related to malocclusion, dysphagia, and facial deformities as well as dentomaxillary disharmony due to maxillary or mandibular hypodevelopment have been described [7,8], as observed in the present case.
Mandibular condyle involvement with GSD may mimic the signs of temporomandibular joint dysfunction or auricular pathology. In cases of skull base bone damage, recurrent meningitis, as observed in the present case, has been reported [10].
Almost complete disappearances of the mandible have been reported in the literature [11,12].
GSD is based on a diagnosis of exclusion, following the elimination of other causes of osteolysis such as malignant (metastases and sarcomas), inflammatory, infectious (osteomyelitis), or endocrine (hyperparathyroidism) pathologies [7,10].
Severe complications of GSD including paraplegia or death in cases of osteolysis with vertebral involvement [4,7]; respiratory failure, hemothorax, or chylothorax in the cases of chest injury; or bacterial meningitis in cases of osteomeningeal breccia [2,12] are rare. In 16% of cases, this disease is fatal [13].
Due to the rarity of GSD, there exists no therapeutic consensus.
Surgical treatments include the resection of the affected bone using bone graft, free-flap, or prosthetic reconstruction; however, these are unreliable over long periods because the disease occasionally recurs on the grafted bone [2,6].
Although radiotherapy facilitates local control by sclerosing the area, the possible sequelae, particularly in the facial bones (osteonecrosis, radiation-induced tumors in young patients), must be considered [6].
The use of bisphosphonates (BPs) appears promising because of their action on osteoclast metabolism. Although the presence of osteoclasts in osteolytic lesions is not systematic, BPs inhibit angiogenesis by inducing endothelial cell apoptosis and thus can control osteolytic lesions [8,9,14].
The administration of BP in children remains rare and limited to specific indications: primary osteoporosis (osteogenesis imperfecta), secondary osteoporosis (corticosteroid-induced), hypercalcemic disorders (malignant hypercalcemia), malignant tumors, or rare bone pathologies (fibrous bone dysplasia, McCune–Albright syndrome, chronic recurrent multifocal osteomyelitis, or GSD) [15].
To date, no cases of BP-related jaw osteonecrosis in the pediatric population have been reported [16–20]. In adults, BP-related osteonecrosis of the jaw is a well-known adverse effect.
Interferon α reduces VEGF activity and appears as a good alternative to the use of BPs [3,10,14].
The evolution of the disease remains difficult to predict. In most cases, bone resorption spontaneously ceases after a variable number of years, regardless of treatment.
Bhatt et al. (2014) provided recommendations regarding the oral surgical management of children who were currently undergoing treatment or were previously treated with BP. In particular, it focuses on the importance of good oral hygiene and the education of the child and his or her parents. Dental avulsions must be performed before treatment is started [17].
For patients who received BP for a short period of time, a latency period of 2 years should be observed after treatment discontinuation before considering surgical interventions. Indeed, the growth and bone turnover of children in the middle of a growth period reportedly increase by 30% within the first 2 years compared with the increase of 10% in children at the end of the growth period [17] (Fig. 6). In the present case, the surgery was performed 7 years after the treatment was discontinued, which was initiated before the patient reached his peak growth rate.
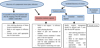 |
Fig. 6 Surgical management pathway for pediatric patients receiving or having received BP (after Bhatt et al. (2014)). |
Conclusion
GSD is a rare disease that frequently affects the facial region and can result in mobility, ectopia, and loss of teeth as well as progressive bone lesions (mostly mandibular lesions) that can lead to the complete disappearance of the bone. It is a pathology that is induced following massive osteolysis without any other objective cause.
To date, a consensus regarding the treatment of this pathology is lacking. Although medical therapeutics appear promising, studies involving clinical case series are required to develop substantiated therapeutic recommendations.
Conflicts of interest
The authors declare that they have no conflicts of interest in relation to this article.
References
- Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am 1955;985:1004. [Google Scholar]
- Gondivkar SM, Gadbail AR. Gorham-Stout syndrome: a rare clinical entity and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;41:48. [Google Scholar]
- Escande C, Schouman T, Françoise G, Haroche J, Ménard P, Piette J-C, et al. Histological features and management of a mandibular Gorham disease: a case report and review of maxillofacial cases in the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;30:37. [Google Scholar]
- Dellinger MT, Garg N, Olsen BR. Viewpoints on vessels and vanishing bones in Gorham-Stout disease. Bone 2014;47:52. [Google Scholar]
- Zhang S, Wu D, Shi L, Zhang Y, Long K, Fan Y, et al. Gorham disease of the mandible: a report of Two cases and a literature review. Oral Surg Oral Med Oral Pathol Oral Radiol 2019;71:76. [Google Scholar]
- Al-Jamali J, Glaum R, Kassem A, Voss PJ, Schmelzeisen R, Schön R. Gorham-Stout syndrome of the facial bones: a review of pathogenesis and treatment modalities and report of a case with a rare cutaneous manifestations. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;23:29. [Google Scholar]
- Holroyd I, Dillon M, Roberts GJ. Gorham's disease: a case (including dental presentation) of vanishing bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;125:9. [Google Scholar]
- Qu L, Cai X, Wang B. Diagnosis and Treatment of Gorham-Stout Disease in Maxillofacial Regions. J Craniofac Surg 2018;460:1. [Google Scholar]
- Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, Andrade ES de S. Use of zoledronic acid in the treatment of Gorham's disease. Int J Pediatr Otorhinolaryngol 2010;319:22. [Google Scholar]
- Galiay L, Simon F, Lévy R, Couloigner V, Donadieu J, Toubiana J, et al. Temporomandibular joint anomalies in pediatric craniofacial Gorham-Stout disease. J Cranio-Maxillo-fac Surg Off Publ Eur Assoc Cranio-Maxillo-fac Surg 2018;1179:84. [Google Scholar]
- Huang Y, Wang L, Wen Y, Zhang Q, Li L. Progressively bilateral resorption of the mandible. J Cranio-Maxillo-fac Surg Off Publ Eur Assoc Cranio-Maxillo-fac Surg 2012;174:177. [Google Scholar]
- Evrenos MK, Özkaya M, Yaman M, Proff LY. Case report: gorham-stoute syndrome with involvement of majority of mandible, and partial maxillary, temporal and zygomatic bones. J Maxillofac Oral Surg 2016;335:8. [Google Scholar]
- Gulati U, Mohanty S, Dabas J, Chandra N. ≪ Vanishing Bone Disease » in maxillofacial region: a review and our experience. J Maxillofac Oral Surg 2015;548:57. [Google Scholar]
- Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol 2010;579:84. [Google Scholar]
- Baroncelli GI, Bertelloni S. The use of bisphosphonates in pediatrics. Horm Res Paediatr 2014;290:302. [Google Scholar]
- Hernandez M, Phulpin B, Mansuy L, Droz D. Use of new targeted cancer therapies in children: effects on dental development and risk of jaw osteonecrosis: a review. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol 2017;321:6. [Google Scholar]
- Bhatt RN, Hibbert SA, Munns CF. The use of bisphosphonates in children: review of the literature and guidelines for dental management. Aust Dent J 2014;9:19. [Google Scholar]
- Brown JJ, Ramalingam L, Zacharin MR. Bisphosphonate-associated osteonecrosis of the jaw: does it occur in children? Clin Endocrinol (Oxf). 2008;863:7. [Google Scholar]
- Chahine C, Cheung MS, Head TW, Schwartz S, Glorieux FH, Rauch F. Tooth extraction socket healing in pediatric patients treated with intravenous pamidronate. J Pediatr 2008;719:20. [Google Scholar]
- Malmgren B, Aström E, Söderhäll S. No osteonecrosis in jaws of young patients with osteogenesis imperfecta treated with bisphosphonates. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol 2008;196:200. [Google Scholar]
All Figures
 |
Fig. 1 Pelvis radiograph showing the bone involvement of the Gorham Stout Disease at the right iliac crest and femur (arrows). |
| In the text | |
 |
Fig. 2 Pre operative orthopantomogram. |
| In the text | |
 |
Fig. 3 Frontal teleradiograph. |
| In the text | |
 |
Fig. 4 Intra oral intraoperative photograph showing 47 48 position (arrow). |
| In the text | |
 |
Fig. 5 Intra oral intraoperative photograph showing inferior alveolar nerve at the bottom of the teeth sockets (arrow). |
| In the text | |
 |
Fig. 6 Surgical management pathway for pediatric patients receiving or having received BP (after Bhatt et al. (2014)). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


