| Issue |
J Oral Med Oral Surg
Volume 24, Number 1, January 2018
|
|
|---|---|---|
| Page(s) | 36 - 39 | |
| Section | Cas clinique et revue de la littérature / Up-to date review and case report | |
| DOI | https://doi.org/10.1051/mbcb/2017025 | |
| Published online | 25 May 2018 | |
Up-to Date Review And Case Report
First-Bite syndrome and Eagle syndrome
Department of Oral Surgery,
3 Chemin des Maraîchers,
31400
Toulouse, France
* Correspondence: benat.g@chu-toulouse.fr
Received:
5
June
2017
Accepted:
19
October
2017
Introduction: Eagle Syndrome (ES) is caused by the ossification/calcification of the stylohyoid ligament and is associated with many different symptoms such as otalgia, restricted mouth opening, or an intrapharyngeal foreign body sensation. First-bite syndrome (FBS) is characterized by pain in the parotid and retromandibular region, when taking the first bite in a meal and occurs more or less invariably. Observation: A 50-year-old female patient presented complaining of right retromandibular pain, that irradiated to the right side of the mandible and right shoulder, only when eating for the first time a day and at the first bite. Medical history and clinical examination did not reveal any signs of cervical surgery or cervical trauma. Palpation was painful at a specific point in right retromandibular point region, the rest of the intraoral and extraoral examinations were normal. The right and left condylar x-rays (open mouth and closed mouth) revealed an elongation of both the right and left stylohyoid ligaments. Discussion: Usually, FBS occurs after cervical surgery, for example after resection of the stylohyoid ligament for ES. Our case report shows, on the contrary, FBS that was associated with ES. The pathophysiological explanation of FBS depends on an irritative or traumatic factor in the sympathetic nerve fibers of the parotid gland. Conclusion: This association allows us to present both syndromes and to carry out an up to date pathophysiological examination and therapeutic proposals concerning FBS.
Key words: eagle syndrome / first-bite syndrome
© The authors, 2018
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Retromandibular cervical pain secondary to the elongation and ossification of the stylohyoid ligament (SHL) was officially described as a syndrome for the first time by Eagle in 1937 [1]. The pathogenesis of Eagle Syndrome (ES) is still misunderstood, and several theories have been proposed for its etiology. The most widely accepted theory for ES following trauma or surgery of the paracervical region is the ossification of SHL involving the compression of adjacent anatomical structures (nerves, vessels, muscles) during mobilization. This triggers pain and various symptoms such as earache, dysgeusia, limitation of mouth opening, an intrapharyngeal foreign body sensation, and limitation of cervical rotation [2]. The ossification of SHL is most often bilateral with unilateral symptomatology. The ossification of SHL is found in approximately 4.2% of the general population and becomes symptomatic in only 4% of these cases, with a female predominance (sex ratio 60%) [3]. First-bite syndrome (FBS) was first described in 1986 by Haubrich [4] and specified by Neterville et al. [5] in 1998. It is characterized by pain in the parotid and retromandibular regions, which is consistently triggered at the time of the first bite of meals. The pathogenesis of this syndrome is poorly understood. The symptomatology described appears after surgery to the paracervical region or during when a tumor develops in this anatomical region. It would appear that FBS is secondary to loss of sympathetic nerve function in the ipsilateral parotid gland. This loss of nerve function is responsible for the denervation of sympathetic receptors of the myoepithelial parotid cells. These cells also have parasympathetic receptors, which become hypersensitive to a parasympathetic stimulation, resulting in intense maximum contraction when taking the first bite, which subsequently induces head and neck pain and/or cramps [6].
Observation
A 50-year-old patient presented to our consultation for pain in the right retromandibular region and right shoulder only when taking her first bite in the morning and sometimes at lunch. These episodes, which occurred over the past 3 months, were spaced apart at first. However, they started occurring daily, resulting in a significant impact on her quality of life. The patient had so far taken no analgesic or anti-inflammatory medicines, adapting her diet to the intensity of the pain, frequently forcing her to stop eating altogether. Questioning revealed no particular antecedents, including cervical surgery or cervical trauma. The cervicofacial clinical examination found tenderness at a specific point in the right retromandibular region, and the rest of the intraoral and extraoral examinations were normal. The oral examination found a good oral state, a lack of dental pain, absence of mucosal lesions, normal salivary flow without palpable sialoliths, absence of temporomandibular dysfunction, and a normal mouth opening without cracking or temporomandibular joint pain. There was no hypertrophy of the masseter muscles, which were soft on palpation, and signs suggesting bruxism were absent. The otorhinolaryngological examination noted an absence of earache or atrial flow, a lack of sinus pathology. Cervical examination did not reveal lymphadenopathy, palpable masses, or cutaneous inflammation or edema. From an orthopedic point of view, neck, and right shoulder mobility were normal. Finally, the neurological examination did not show any damage to the cranial nerves. Further radiological examinations were performed. The orthopantomogram showed no dentomaxillary anomalies (Fig. 1). The right and left condylar images in the open mouth and closed mouth positions showed elongation and ossification of the right and left SHLs (Fig. 2), which were both classified as ES type 1D according to the Langlais classification (Figs. 3 and 4) [7] Cervicofacial computed tomography with contrast scan did not reveal any expansive processes or vascular anomalies. We eliminated all diagnostic hypotheses (sialolithiasis, salivary pathologies, expansive processes, temporomandibular dysfunction, dental pathology, otorhinolaryngological disorders, neurological disorder). Considering that the patient experienced unilateral pain in the retromandibular region triggered when taking first bite and because characteristic SHL findings on radiography, FBS secondary to ES was diagnosed.
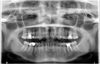 |
Fig. 1 Orthopantomogram of the patient. |
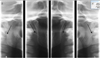 |
Fig. 2 Condylar images in the open and closed mouth positions, showing the ossification and elongation of the right and left SHLs (black arrows). |
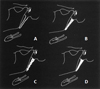 |
Fig. 4 Calcification of SHL: A. Calcified contour–B. Partially Calcified–C. Nodular calcifications–D. Complete calcification [7]. |
Comments
FBS is described as occurring following a surgical procedure in the paracervical region because of a lesion of the sympathetic nerve fibers innervating the parotid gland [6]. ES is described following SHL ossification. A single case report in the literature mentions the combination of the two syndromes, but in reverse order to the one reported here. In the article by Cerna et al. [8], LSH resection to treat ES was responsible for FBS. Their patient had received medical treatment with carbamazepine (2 × 400 mg/day for 2 years) with a complete resolution of symptoms after 28 months. Now, the main treatment for FBS is the intraparotid injection of botulinum toxin (BT) based on different treatment procedures. Ali et al. injected 75 IU of diluted BT in 2 ml of physiological saline solution in a single session in a diffuse manner in the parotid gland with particular attention paid to the most painful area; the patient reported no pain after 48 h with no symptom recurrence at 10 weeks [9]. Ali et al. expected a recurrence of symptoms 4–6 months after the injection and scheduled the patient for a new BT injection. Lee et al. divided BT injection into three doses of 11 IU of BT each, diluted in a physiological serum solution, for a total of 33 IU; they observed a significant difference in pain during eating and quality of life at 3 months in five patients [6]. Costaes-Marcos et al treated five patients with three concurrent injections of 10 IU of BT injected into the parotid gland. Injections were repeated in three patients (50 IU after 7 months in one patient, and 50 IU after 6 weeks in two patients). In four patients, the pain decreased (intensity from severe to moderate; VAS 8–10 to 2–6) 6 months after the injection, only one patient continued to suffer from intense pain. No patient was completely free of symptoms. However, they concluded that the BT injection was an effective treatment method that allowed for improved symptoms and quality of life without significant side effects. However, it was necessary to administer injections every 4–6 months because of the transient effect of BT, until the symptoms disappeared [10]. Ghosh et al injected a series of five patients with 10–40 IU of BT in two to seven sites in the parotid gland. They repeated the injections every 4 months until the symptoms had gone, which occurred within an average period of 16–17 months (10–28 months), concluding that BT injection was a noninvasive and effective technique [11]. It should therefore be concluded, in the light of the various published protocols, that injections of 30–50 IU of Type A BT every 4–6 months in the parotid gland in case of FBS until the symptoms have disappeared is the best treatment option. This requires long-term follow-up of patients to watch out for any partial or total relapses.
In our case, FBS led us to the diagnosis of ES. The external carotid artery supplies both arterial blood sympathetic nerve bundles of the parotid gland [12]. Because of the proximity of the anatomical structures (Figs. 5 and 6) [13], ossified SHL can cause arterial dissection of the external carotid artery [14], which damages the sympathetic nerve fibers, thus causing FBS. This is, to the best of our knowledge, the first case report of its kind described in the literature where FBS was provoked by ES.
 |
Fig. 6 Anatomical relationships between SHL and the external carotid artery. ECA(ACE): external carotid artery − M: mastoid − SP(AS): styloid process − ICA(ACI): internal carotid artery − IJV(VJI): internal jugular vein [13]. |
Conclusion
When FBS occurs without a history of cervical trauma, physicians should look for an undiagnosed ES. The treatment of FBS is based on injections of 30–50 IU of Type-A BT in the parotid gland, to be repeated every 4–6 months until the symptoms disappear.
References
- Eagle WW. Elongated styloid process. Report of two cases. Arch Otolaryngol 1937;25:584–587. [CrossRef] [Google Scholar]
- Sudrat Y, Teitelbaum J, Antoine L, Mondié JM, Baudet-Pommel M. Syndrome d'Eagle: à propos d'un cas avec calcifications multiples. Médecine Buccale Chir Buccale 2008;14:97–102. [CrossRef] [EDP Sciences] [Google Scholar]
- Scanteie R, Pasquet G. Imagerie du système stylo-hyoïdien: variabilité chez l'homme. Actual Odontostomatol 2012;260:365–372. [Google Scholar]
- Haubrich WS. The first-bite syndrome. Henry Ford Hosp Med J 1986;34:275–278. [PubMed] [Google Scholar]
- Netterville JL, Jackson CG, Miller FR, Wanamaker JR, Glasscock ME. Vagal Paraganglioma. Arch Otolaryngol Neck Surg 1998;124:1133–1140. [CrossRef] [Google Scholar]
- Lee B-J., Lee J-C., Lee Y-O., Wang S-G., Kim H-J. Novel treatment of first bite syndrome using Botulinum toxin type A. Head Neck 2009;31:989–993. [CrossRef] [PubMed] [Google Scholar]
- Langlais RP, Miles DA, Van Dis ML. Elongated and mineralized stylohyoid ligament complex: a proposed classification and report of a case of Eagle's syndrome. Oral Surg Oral Med Oral Pathol 1986;61:527–532. [CrossRef] [PubMed] [Google Scholar]
- Cernea CR, Hojaij FC, De Carlucci D, Plopper C, Vanderley F, Guerreiro CM, et al. First-bite syndrome after resection of the styloid process. Laryngoscope 2007;117:181–182. [CrossRef] [PubMed] [Google Scholar]
- Ali MJ, Orloff LA, Lustig LR, Eisele DW. Botulinum toxin in the treatment of first bite syndrome. Otolaryngol Head Neck Surg 2008;139:742–743. [CrossRef] [PubMed] [Google Scholar]
- Costales-marcos M, López F, Fernández-va L. Tratamiento con toxina botulínica del síndrome del primer mordisco. Acta Otorrinolaringol Española 2017;68:284–288. [CrossRef] [Google Scholar]
- Ghosh A, Mirza N. First bite syndrome: our experience with intraparotid injections with botulinum toxin type A. Laryngoscope 2016;126:104–107. [CrossRef] [PubMed] [Google Scholar]
- Avinçsal MÖ, Hiroshima Y, Shinomiya H, Otsuki N, Nibu K. First bite syndrome − a 11-year experience. Auris Nasus Larynx 2016;44:302–305. [PubMed] [Google Scholar]
- Dulguerov P, Kohler R, Becker M. Carotidynie et syndrome d'Eagle: deux syndromes classiques à redécouvrir. Rev Med Suisse 2011;7:1929–1934. [PubMed] [Google Scholar]
- Bizet A, Margottin C, Lagarde A, Malard O, Corre P, L esclous P. Prise en charge chirurgicale par voie endobuccale d'une patiente atteinte d'un syndrome d'Eagle: cas clinique et revue de la littérature. Médecine Buccale Chir Buccale 2016;22:63–75. [Google Scholar]
All Figures
 |
Fig. 1 Orthopantomogram of the patient. |
| In the text | |
 |
Fig. 2 Condylar images in the open and closed mouth positions, showing the ossification and elongation of the right and left SHLs (black arrows). |
| In the text | |
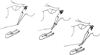 |
Fig. 3 Type I: SHL elongated-type II: SHL Pseudoarticulated-type III: SHL segmented [7]. |
| In the text | |
 |
Fig. 4 Calcification of SHL: A. Calcified contour–B. Partially Calcified–C. Nodular calcifications–D. Complete calcification [7]. |
| In the text | |
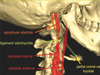 |
Fig. 5 Anatomy of the stylohyoid complex [13]. |
| In the text | |
 |
Fig. 6 Anatomical relationships between SHL and the external carotid artery. ECA(ACE): external carotid artery − M: mastoid − SP(AS): styloid process − ICA(ACI): internal carotid artery − IJV(VJI): internal jugular vein [13]. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


