| Issue |
J Oral Med Oral Surg
Volume 28, Number 1, 2022
|
|
|---|---|---|
| Article Number | 9 | |
| Number of page(s) | 6 | |
| DOI | https://doi.org/10.1051/mbcb/2021048 | |
| Published online | 21 February 2022 | |
Case Report
Central giant cell granuloma of the mandibular condyle: additional case and literature review
1
University of Lille, Department of Oral and Maxillofacial Surgery, CHU Lille, 59000 Lille, France
2
University of Lille, Department of Oral and Maxillofacial Surgery, CHU Lille, INSERM U 1008, Controlled Drug Delivery Systems and Biomaterials, 59000 Lille, France
* Correspondence: lucas.martiflich@chu-angers.fr
Received:
6
April
2021
Accepted:
18
October
2021
Introduction: Central giant cell granuloma (CGCG) of the jaws is not a common lesion. Only five cases are reported in the mandibular condyle. Observation: A 25 year-old male presented with preauricular swelling and a premature occlusal contact on the molars. The lesion had radiological features of aggressiveness and a high metabolic uptake. Initial biopsy was misleading. The lesion was treated surgically by resection. Discussion: Histologically, CGCG are very similar to other giant cell lesions such as GCT (Giant cell Tumor) or BTH (brown tumor of hyperparathyroidism). The standard treatment is surgical either by curettage or resection. Only 6 cases have been described in the literature, including this one. The diagnosis is difficult, relying on a bundle of clinical, radiological and histological arguments. However, radical surgery should be performed to avoid the tumor recurrence. The genetic mutations associated with CGCG (notably TRPV4 and RAS pathway) may explain why this tumor is mostly found in the dental part of the jaws and only rarely in the mandibular condyle.
Key words: Central giant cell granuloma / mandibular condyle / bone tumor / benign bone lesion / recurrence rate
© The authors, 2022
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Central giant cell granuloma (CGCG) of the jaws is not a common lesion with only 2270 cases reported in the literature in 2018 [1], the mandibular condyle is seldom affected with only 5 cases reported [2]. The CGCG is more prevalent in females (1.56 females for 1 male), and in young patients with a highest prevalence in the second and third decade of life (mean age of 25.8 years) [1]. The initial description was made by Jaffe in 1953 [3]. It is a benign lesion of the giant cell lesion family, closely related to giant cell tumors (GCT) and brown tumors of hyperparathyroidism (BTH). Two forms are described: aggressive and non-aggressive based on clinical and radiological examination, the aggressive form being associated with a higher recurrence rate. The lesion is characterized by multinucleated osteoclast-like giant cells intermingled with mononuclear spindle cells and hemorrhagic foci with hemosiderin deposition according to the World Health Organization [4]. Etiology is unknown, however previous local trauma, inflammatory lesion or genetic predisposition could be involved. The standard treatment is surgical either by curettage or resection [5] but medication-based treatment using corticosteroids, calcitonin or interferon α have been described, albeit with a lower success rate. The first cases of CGCG located in the mandibular condyle were described in 1978 [6,7].
We report here an exceptionally rare case of CGCG of the mandibular condyle, with a complete literature review.
Observation
A 25 year-old male student, with no medical history, was referred to our Department for a painless right preauricular swelling. It was associated with a premature occlusal contact on the right molars. The swelling was first noted three months ago and was slowly swelling. The physical examination did not reveal other abnormalities besides the facial swelling, there was no facial palsy and no neck lymphadenopathy.
Computed tomography (CT) scanning exhibited a large radiolucent lesion with marked cortical thinning of the right condyle (Fig. 1). Magnetic resonance imaging (MRI) revealed a multicystic lesion of the right mandibular condyle with local aggressive characteristics (large swelling, rapid rate of growth and bone cortical resorption) (Fig. 2). There was no soft tissue involvement. Positron Emission Tomography (PET) scanning showed a high metabolic feature of the lesion with a standardized uptake value (SUV) peak = 9.5 (Fig. 3).
A surgical biopsy of the lesion was performed under general anesthesia through a preauricular approach. Pathological examination found focal hemosiderin deposition and small, unevenly distributed clusters of giant cells, which seemed in favor of an aneurysmal bone cyst (ABC).
Due to radiological features of aggressiveness of the lesion and its high metabolic uptake, it was decided to perform a surgical resection. The biopsy was indeed in favor of an ABC but other diagnosis such as malignant lesions could not be excluded. Therefore, condylar resection was performed through a combined preauricular and modified Risdon (angulo-mandibular) approaches. The tumor could be removed completely, and the surrounding soft tissues were intact. Condylar reconstruction was performed simultaneously with a costochondral graft harvested on the 6th right rib (Fig. 4). Arch bars were placed intraoperatively for maxillomandibular fixation. The maxillomandibular fixation was maintained 10 days, then nocturnal fixation was performed until removal of the arch bars at 6 weeks following the surgery. Final pathological examination concluded to a central giant cell granuloma. There were no postoperative complications.
There were follow-up visits at 15 days, 6 weeks, 3 months, 6 months and 1 year postoperatively. At each visit, a clinical examination associated with an orthopantomogram was performed. A CT-scan was performed at 3 months postoperatively. The patient is currently recurrence-free but still under surveillance.
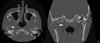 |
Fig. 1 CT-Scan axial (A) and coronal (B) view showing the radiolucent lesion with osteolysis of the right mandibular condyle (arrow). |
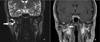 |
Fig. 2 MRI coronal view, T2 sequence (A), T1 with gadolinium injection (B) showing the lesion of the right mandibular condyle (arrow). |
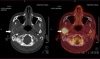 |
Fig. 3 PET-Scanner, axial view showing high metabolic feature of the right mandibular condyle lesion with a standardized uptake value peak of 9.5. |
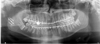 |
Fig. 4 3 months postoperative CT-Scan showing the integration of the costochondral graft. |
Discussion
Head and neck CGCG are mostly localized in the dental part of the mandible [1], the condylar localization is exceptionally rare as only 5 cases were reported in the literature as shown in Table I. Patient with CGCG of the mandibular condyle were aged of 15 years old to 60 years old (mean 36 years old) at time of the diagnosis. CGCG can be classified in aggressive and non-aggressive lesions using the following criteria (adapted to the dental part of the mandible): >5 cm, rapid growth, bone cortical thinning, bone cortical perforation, dental rhizalysis and dental mobility [8]. When 3 of these criteria are met, the lesion can be considered as aggressive. Aggressive tumors are encountered in younger patients and tend to recur more frequently [8,9].
The diagnosis relies on a bundle of clinical, radiological and histological arguments. However, histological examination is difficult, because CGCG are very similar to other giant cell lesions, such as GCT or BTH, which can be differentiated using p63 marking (negative in CGCG and positive in GCT or BTH) or H3G34W (immunohistochemical marker for H3F3A mutation, which is positive in GCT) [10] or other giant cell-like tumors such as aneurysmal bone cyst, or associated with inherited syndromes, such as cherubism or Noonan syndrome [11].
Etiology of CGCG is currently unknown. Role of local trauma or inflammation are often evoked [12] but a genetic origin has also been discussed [13]. H3F3A is coding for the amino-acid histone 3.3. H3F3A mutations are involved in a variety of bone and cartilage tumors, such as GCT or chondroblastoma, however the repercussions of these mutations are not fully yet known. Chondroblastoma and GCT of bone may derive from a common precursor cell, the differentiation of which is determined by specific histone 3.3 variants [14].
Contrarily, in CGCG, no H3F3A mutations have been described. Molecular findings showed recently that gain-of-function mutation identified in CGCG suggested a pathogenetic relationship to non-ossifying fibroma (NOF) of bone [15]. These gain-of-function mutations notably include TRPV4, which promotes osteoclastic cellular differentiation, modulate vascular function and inhibit osteoclast apoptosis. They also include KRAS mutations and FGFR1, which both regulate bone growth and remodeling [16]. This could explain the frequency of CGCG in the dental part of the jaw due to high turnover of the alveolar bone. CGCG and NOF belong to the phenotypic spectrum of specific RASopathies [17]. CGCG most probably like NOF consists of a neoplastic population initiated by blood-borne recruitment [18]. The poorer vascularization of the mandibular condyle compared to the alveolar bone may be another hypothesis of the preferential localization of the CGCG in the dental part of the jaws.
The standard treatment is surgical either by curettage or resection [1,5,19]. CGCG is a benign lesion but is associated with a high recurrence rate, especially in aggressive lesions [8]. Resection has the lowest recurrence rate (9% for aggressive CGCG and 0% for non-aggressive CGCG) [1,5]. However, resection causes a higher morbidity than curettage, which is associated with a higher recurrence rate (37% for aggressive CGCG and 9% for non-aggressive CGCG) [1,5,20]. Medication-based treatment using intralesional corticosteroids, systemic calcitonin or interferon α, have been described albeit with a lower success rate: complete regression in 58.6%, 51.3% and 0% of the cases respectively and partial regression in 28.3% for corticosteroids or in 30.8% for calcitonin. Other therapeutics are described in the literature, such as corticoid injection, calcitonin therapy [20]. Therefore, medical treatment seems best in association with the surgical treatment to reduce its size and limit the morbidity. Enucleation has been performed successfully in the literature, however with a follow-up of 1 year or 6 months when disclosed. Late recurrence may have happened, as in the study with a follow-up of 4 years, a secondary resection was performed after initial enucleation. We decided to perform a resection in our case due signs of aggressiveness on imaging, hypermetabolic fixation on PET-scanner imaging, and uncertain biopsy result. Surgical resection was favored as malignant lesions could not be ruled out. Furthermore, secondary fracture of the mandibular condyle could occur following curettage leading to malocclusion, whereas immediate reconstruction using costochondral grafting allows occlusal restoration.
CGCG of the mandibular condyle are extremely rare. Only 6 cases have been described in the literature, including this one. The diagnosis is difficult and histological results can be misleading. The treatment is surgical but medication-based treatments have been described with a partial success. The genetic mutations associated with CGCG (notably TRPV4 and RAS pathway) may explain why this tumor is mostly found in the dental part of the jaws and only rarely in the mandibular condyle.
Literature review summary (OPG for orthopantomogram, CGCG for central giant cell granuloma, ABC for aneurismal bone cyst).
Conflicts of interest
The authors declare that there is no conflict of interest.
Funding
This research did not receive any specific funding.
Ethical approval
Ethical approval was not required.
Informed consent
The authors declare that informed consent not required.
Authors contribution
R. Nicot: Conceptualization, Methodology. L. Marti-Flich: Writing original draft. L. Marti-Flich: Visualization, Investigation. R. Nicot: Supervision. M. Schlund: Writing- Reviewing and Editing.
References
- Chrcanovic BR, Gomes CC, Gomez RS. Central giant cell lesion of the jaws: an updated analysis of 2270 cases reported in the literature. J Oral Pathol Med 2018;47:731–739. [CrossRef] [PubMed] [Google Scholar]
- Bocchialini G, Salvagni L, Guerini A, Castellani A. Central giant cell granuloma of the mandibular condyle: a rare case and a literature review. Heliyon 2020;6:e03085. [CrossRef] [PubMed] [Google Scholar]
- Jaffe HL. Giant-cell reparative granuloma, traumatic bone cyst, and fibrous (fibro-osseous) dysplasia of the jawbones. Oral Surg Oral Med Oral Pathol 1953;6:159–175. [CrossRef] [PubMed] [Google Scholar]
- Speight PM, Takata T. New tumour entities in the 4th edition of the World Health Organization Classification of Head and Neck tumours: odontogenic and maxillofacial bone tumours. Virchows Arch 2018;472:331–339. [CrossRef] [PubMed] [Google Scholar]
- Jadu FM, Pharoah MJ, Lee L, Baker GI, Allidina A. Central giant cell granuloma of the mandibular condyle: a case report and review of the literature. Dentomaxillofac Radiol 2011;40:60–64. [CrossRef] [PubMed] [Google Scholar]
- Shensa DR, Nasseri S. Central giant cell reparative granuloma of the mandibular condyle. J Oral Surg 1978;36:642–643. [PubMed] [Google Scholar]
- Tasanen A, von Konow L, Nordling null. Central giant-cell lesion in the mandibular condyle. Report of a case. Oral Surg Oral Med Oral Pathol 1978;45:532–539. [CrossRef] [PubMed] [Google Scholar]
- Chuong R, Kaban LB, Kozakewich H, Perez-Atayde A. Central giant cell lesions of the jaws: a clinicopathologic study. J Oral Maxillofac Surg 1986;44:708–713. [Google Scholar]
- Abu-El-Naaj I, Ardekian L, Liberman R, Peled M. Central giant cell granuloma of the mandibular condyle: a rare presentation. J Oral Maxillofac Surg 2002;60:939–941. [CrossRef] [PubMed] [Google Scholar]
- Schlund M, Roland-Billecart T, Aubert S, Nicot R. Tumeurs de l'articulation temporomandibulaire − revue de la littérature. Bull Cancer 2020;107:1186–1198. [CrossRef] [PubMed] [Google Scholar]
- Lee JS, Tartaglia M, Gelb BD, Fridrich K, Sachs S, Stratakis CA, et al. Phenotypic and genotypic characterisation of Noonan-like/multiple giant cell lesion syndrome. J Med Genet 2005;42:e11. [CrossRef] [PubMed] [Google Scholar]
- Eisenbud L, Stern M, Rothberg M, Sachs SA. Central giant cell granuloma of the jaws: experiences in the management of thirty-seven cases. J Oral Maxillofac Surg 1988;46:376–784. [CrossRef] [PubMed] [Google Scholar]
- Teixeira RC, Horz HP, Damante JH, Garlet GP, Santos CF, Nogueira RLM, et al. SH3BP2-encoding exons involved in cherubism are not associated with central giant cell granuloma. Int J Oral Maxillofac Surg 2011;40:851–855. [CrossRef] [PubMed] [Google Scholar]
- Behjati S, Tarpey PS, Presneau N, Scheipl S, Pillay N, Van Loo P, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet 2013;45:1479–1482. [CrossRef] [PubMed] [Google Scholar]
- Cleven AHG, Schreuder WH, Groen E, Kroon HM, Baumhoer D. Molecular findings in maxillofacial bone tumours and its diagnostic value. Virchows Arch 2020;476:159–174. [CrossRef] [PubMed] [Google Scholar]
- Gomes CC, Diniz MG, Bastos VC, Bernardes VF, Gomez RS. Making sense of giant cell lesions of the jaws (GCLJ): lessons learned from next‐generation sequencing. J Pathol 2020;250:126–133. [CrossRef] [PubMed] [Google Scholar]
- Bovée JV, Hogendoorn PC. Non-ossifying fibroma: A RAS-MAPK driven benign bone neoplasm. J Pathol 2019;248:127–130. [CrossRef] [PubMed] [Google Scholar]
- Faverani LP, Ferreira S, Ferreira GR, Coléte JZ, Aranega AM, Garcia Júnior IR. Central giant cell granuloma in pediatric maxilla: surgical management. J Craniofac Surg 2014;25:e344–346. [CrossRef] [PubMed] [Google Scholar]
- Nicolai G, Lorè B, Mariani G, Bollero P, De Marinis L, Calabrese L. Central giant cell granuloma of the jaws. J Craniofac Surg 2010;21:383–386. [CrossRef] [PubMed] [Google Scholar]
- Harris M. Central giant cell granulomas of the jaws regress with calcitonin therapy. Br J Oral Maxillofac Surg 1993;31:89–94. [CrossRef] [PubMed] [Google Scholar]
All Tables
Literature review summary (OPG for orthopantomogram, CGCG for central giant cell granuloma, ABC for aneurismal bone cyst).
All Figures
 |
Fig. 1 CT-Scan axial (A) and coronal (B) view showing the radiolucent lesion with osteolysis of the right mandibular condyle (arrow). |
| In the text | |
 |
Fig. 2 MRI coronal view, T2 sequence (A), T1 with gadolinium injection (B) showing the lesion of the right mandibular condyle (arrow). |
| In the text | |
 |
Fig. 3 PET-Scanner, axial view showing high metabolic feature of the right mandibular condyle lesion with a standardized uptake value peak of 9.5. |
| In the text | |
 |
Fig. 4 3 months postoperative CT-Scan showing the integration of the costochondral graft. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


