| Issue |
J Oral Med Oral Surg
Volume 26, Number 4, 2020
|
|
|---|---|---|
| Article Number | 45 | |
| Number of page(s) | 11 | |
| Section | Revue de la littérature / Literature review | |
| DOI | https://doi.org/10.1051/mbcb/2020040 | |
| Published online | 11 September 2020 | |
Literature Review
Cancerous lesions in the vicinity of dental implants: a systematic review
1
Faculty of Dentistry, University of Seville, Seville, Spain
2
Faculty of Dentistry, Complutense University of Madrid, Madrid, Spain
3
Department of Dental Clinical Specialties, Faculty of Dentistry, Complutense University of Madrid, Madrid, Spain
* Correspondence: orionsalgado@hotmail.com
Received:
16
April
2020
Accepted:
25
August
2020
Introduction: The massive diffusion of dental implant treatments in the last decades leads to the appearance of complications, most of them inflammatory, although important complications have been described as malignant lesions in the vicinity of dental implants. The objective of this article is to describe the cases described in the literature of oral squamous cell carcinoma (OSCC) or clinical variants and metastases, in the vicinity of dental implants and to analyze the possible etiological agents involved. Material and methods: The criteria used were those described in the PRISMA® Declaration for performing systematic reviews. An electronic search was performed on MEDLINE (via PubMed) using the terms MeSH: “dental implants” AND “squamous cell carcinoma” OR “dental implant complications” AND “squamous cell carcinoma”. Results: Thirty-eight articles describing a total of 76 cases of OSCC or clinical variants, as well as metastasis in the vicinity of dental implants, were included. Conclusions: It is not possible to establish a cause-effect relationship between dental implants and the development of OSCC. Its clinical appearance can be confused with periimplantitis, so that, in cases of sudden onset, which do not respond to conventional treatment and/or have associated alterations in sensitivity, a biopsy should be performed.
Key words: oral squamous cell carcinoma / dental implants / peri-implantitis / dental implant complications
© The authors, 2020
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The sixth most common cancers are located within the head and neck region, 48% of which in the oral cavity, representing 3% of all cancers [1]. 90% of cancers of the oral cavity are histopathologically oral squamous cell carcinomas (OSCC) [2,3], malignant neoplasms derived from the stratified squamous epithelium of the oral mucosa [4–6]. Men are more affected by OSCC than women (the ratio by gender being 1.5:1), especially during their sixties; however, the incidence in people younger than 45 is increasing [7], which could be related to human papilloma virus (HPV) infections.
The pathogenesis of OSCC is a multistep process, beginning with irreversible changes of the squamous epithelium, including epithelial dysplasia and further progression into an intraepithelial neoplasia/in-situ carcinoma [8]. This develops into an invasive carcinoma due to the cumulation of genetic alterations, triggered by prolonged exposure to carcinogens [9]. Risk factors such as the following have been cited as part of its multifactorial aetiology: tobacco smoking and alcohol consumption, precursor lesions or potentially malignant disorders (such as leukoplakias, erythroplakias, erythroleukoplakias and/or oral lichen planus), chronic inflammation due to mechanical irritation (trauma) or bacterial infections (poor oral hygiene and periodontitis), viral infections (such as HPV), genetic predisposition, and/or having received treatment for previous malignant lesions [10–12].
Primary cancers that metastasize to the oral cavity are estimated to represent 1%, and in two thirds of cases, until the metastasis is not diagnosed, primary tumours go unnoticed [5,13]. The most frequent primary tumours that metastasise to the oral cavity in women are those located in the breast (42%), while in men, the most frequent are those in the lung (22.3%) and prostate (12%) [5].
Today, dental implants stand as the most effective treatment option for the rehabilitation of partially dentate and edentulous patients, and in certain cases, they are the only option. However, as the popularity of using dental implants has increased over recent decades, alongside has the number of complications. Although most are inflammatory in nature, other important complications have also been described, such as cases of malignant lesions in close proximity to dental implants. The number of cases cited in the literature over the past decades has notably increased [14]. Bhatavadekar [15] estimated the standardized incidence rate of the risk of suffering OSCC following the placement of a dental implant to be 0.00017 per million inhabitants per year. Despite this being a low figure, it is presumable that the number of cases diagnosed and/or described in the literature is less than the real number of patients affected.
Being familiar with the levels of prevention of OSCC is of utmost importance, being of interest implementing in primary centres control and maintenance prevention initiatives for patients susceptible of suffering of OSCC. Primary prevention is essential, reducing or eliminating the risk factors clearly linked to carcinogenesis, especially tobacco smoking and alcohol consumption, as well as improving diet; and secondary prevention, which involves the early detection and diagnosis of potentially malignant disorders. Tertiary prevention aims to avoid the recurrence or appearance of new primary tumours, as well as reducing morbidity.
The objective of this article is to carry out a systematic review of the existing literature in order to collect the cases of OSCC described or clinical variants, as well as metastasis, in the vicinity of dental implants and analyse the possible etiological agents involved.
Material and methods
Search strategy
An electronic search in MEDLINE (via PubMed), without temporal restriction updated to March 2020, using a combination of the following MeSH terms (Medical Subjects Headings) and Boolean operators, was performed: “dental implants” AND “squamous cell carcinoma” OR “dental implant complications” AND “squamous cell carcinoma”.
The present review followed the PRISMA® Statement guidelines (Preferred Reporting Items for Systematic Reviews and Meta-analysis). The objective of this systematic review was to answer the following “PICO” (P = patient / problem / population; I = intervention; C = comparison; OR = outcome) question (Tab. I).
What is the relationship between peri-implant oral cancer in patients with/without a history of potentially malignant disorders, oral cancer or cancer in other locations?
Moreover, a search in Google Scholar was also carried out, using the references of the articles which complied with inclusion criteria, for publications of potential interest that did not appear in the initial search.
Before the beginning of the study, a consensus was reached among all the authors, and a series of inclusion and exclusion criteria were defined.
PICO question breakdown.
Inclusion criteria
The studies to be selected had to fulfil the following criteria: (a) studies in humans; (b) articles published in English, Spanish or French; (c) systematic reviews with or without meta-analyses; and, (d) case reports and series.
Exclusion criteria
Studies that met the following exclusion criteria were disregarded: (a) experimental laboratory studies; (b) animal studies; (c) studies which main topic was not the description of the presence of metastasis, OSCC or clinical variants in the peri-implant mucosa; (d) duplicate articles; (e) books or book chapters; (f) author comments; and (g) letters to the Editor.
Bias risk assessment
Bias risk assessment was assessed independently by two reviewers (AOSP and VSS). Disagreements were resolved by consensus between the two reviewers or the intervention of a third author (MVMM).
Results
The initial search strategy on MEDLINE via PubMed yielded 232 articles for screening by title and abstract, out of which, under consensus, 160 were excluded due to lack of compliance with inclusion criteria and after duplicates removed. 30 articles were selected for full-text reading, out of which 4 were excluded for not describing malignancies in the peri-implant mucosa in the form of metastasis, OSCC, or other histopathological variants, as well as another case report for not providing sufficient information, resulting in 26 articles obtained from MEDLINE.
The references of the aforementioned articles were analysed for an additional electronic search on Google Scholar, adding 12 more articles that fulfilled inclusion criteria. Finally, 38 articles were included in the present review, describing cases of malignancies in the peri-implant mucosa in the form of metastasis, OSCC, or other histopathological variants (Fig. 1).
In general, the available literature is limited to clinical cases or series of small cases, which represents a limitation for the present study (Tab. II). The 38 included studies describe a total of 76 cases in the peri-implant mucosa, in particular, 69 cases of OSCC [5,6,14–41] (of which 12 were recurrent) [16–18,20,26,27,30,35,39], two lung metastases [36,42] and three breast metastases [13,43,44]. Two cases of verrucous carcinomas were also described [32].
The mean age of the patients was 67.48 ± 9.99 years. More women (N = 47) were affected than men (N = 29). The mandibular arch (N = 67) was more affected than the maxillary arch (N = 7), and in one patient, both were affected [13], especially in posterior areas. In most cases, the implants were restored by overdentures (N = 36) and, to a lesser extent, by fixed prostheses (N = 34). In 6 cases, the authors did not describe restorations [30,32,35].
Practically all patients had intraoral (N = 21) [16,17,20,21, 26–30,32,35,36,38,39] against extraoral cancers (n = 8) [5,9,13,26,42–45], or potentially malignant mucosal disorders (n = 29) such as leukoplakias, erythroleukoplakias or oral lichen planus lesions [6,14,18,20,25–28,32,36–38,40]. In fact, in 5 of the aforementioned cases there was a combination of previous carcinomas and potentially malignant mucosal disorders [20,27,28,32,36,38]. In 9 cases, the authors did not describe this aspect [23,25,35,36,41,46], and only 15 patients without a history of lesions of this type [6,15,19,21,22,24,30–34,47].
In addition, in 18 cases, no risk factors were identified [6,20,22,26,30,32–34,36] and in 27 cases the authors did not mention whether there were any risk factors [5,6,14,16,17,24,25,27,35,36,38,42–46]. Among the remaining 33 cases, the following risk factors were identified: smoking [6,21,25,26,36,37,39] (or being an ex-smoker [18,23,28, 30,32,47]), alcohol consumption [6,9,21,23,29–31,39,47] (or being an ex-alcohol drinker [32]), or both [6,21,23,30–32,39,47]; poor oral hygiene [6,13,15,29,31,47]; trauma to the soft tissues due to poorly adjusted prostheses before implant placement [29,41], or cantilevers of implant-supported prostheses [19]; a history of systemic lupus erythematosus [14]; prolonged treatment with immunosuppressants, more specifically, 3 mg/day of prednisone [40]; and one case of treatment with intravenous bisphosphonates (4 mg of zoledronic acid/month, for 33 months). There were only 4 cases in which there was no history of cancer and/or potentially malignant disorders, nor risk factors [22,32–34].
The most common clinical appearance was a combination of clinical signs described by most authors as periimplantitis, in the form of an exophytic lesion of the peri-implant mucosa, in some cases ulcerated, with spontaneous haemorrhage or on probing, and osteolysis [19,29,32–37,41,46]. One author described a clinically acceptable peri-implant appearance in one patient, while radiographically, there was radiolucency along the length of the implants [44]. Some authors describe associated cervical lymphadenopathies [19,29,32,35–37,41], verrucous carcinomas [32,45], paraesthesia [36,40,43] (including numb chin syndrome [44]), pain [43], lesions that did not respond to conventional treatment [5,9,20–22,35,36] and of rapid evolution [13,18,23,28,32,37,42,45], bone expansion, trismus [36], purulent drainage [43] and mobility of teeth adjacent to the implant [40] or of the implants themselves [13,32]. In one case, the presence of metal filings from the implant in the affected peri-implant mucosa was described [29] and in another, the sudden appearance of cellulitis of the mental nerve area without associated bone lesions that led to the loss of osseointegration of the implant [32].
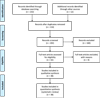 |
Fig. 1 PRISMA® flow diagram of the search processes and results. |
Summary of the cases described in the literature of metastasis, OSCC or clinical variants in the peri-implant mucosa (F, female; M, male; mand, mandible; max, maxilla; BCC, basal cell carcinoma; UNS, unspecified by the authors; OD, overdenture; FP, fixed prosthesis; OSCC, oral squamous cell carcinoma; AP, anatomopathological; VC, verrucous carcinoma; OH, oral hygiene; Tx, treatment; OLP, oral lichen planus).
Discussion
The development of OSCC in proximity to dental implants is a rare event and so far, documented only by case reports or small case series. However, dental implants are becoming an increasingly popular option in the rehabilitation of partially dentate or edentulous patients, therefore, even a phenomenon that until now has been poorly documented can obtain clinical relevance as a consequence of a large number of implants that are placed [6].
It is difficult to establish a causal relation between the placement of a dental implant and the development of a malignant lesion in its vicinity [48], however, several risk factors have been described in the literature:
Relationship between chronic inflammation and cancer
The most plausible theory was described by Virchow in 1863, the first to describe the role of chronic inflammation in cancer [49]. Inflammation is a protective response of the tissues to irritation or infections, but in some cases, inflammation itself can trigger a malignant transformation, while, in other cases, it is activated and modulated by genetic factors and/or carcinogenic risk factors, linked to lifestyle (tobacco and alcohol), mechanical irritants (such as repeated trauma due to ill-fitting prostheses) [10–12], bacterial infections (periodontitis) [6] and even viral infections (HPV) [10–12]. The inflammatory response contributes to tumour progression, increasing the proliferation and survival of malignant cells, stimulating neoangiogenesis, and reducing antitumor immunity [50,51]. Peri-implant inflammation initially affects the peri-implant soft tissues, causing mucositis. When this inflammation is prolonged over time, it becomes chronic and causes peri-implant bone loss [18,39,40]. If this inflammation is sustained, it may have the sufficient potential to induce cell proliferation and prolong cell survival by activating oncogenes and inactivating tumour suppressor genes, causing genetic instability and an increased risk of cancer. In addition, certain inflammation pathways have an effect on carcinogenesis [4,6,21,52]. On the other hand, the peri-implant mucosa is a tissue that suffers chronic inflammation. In this regard, Silva et al. [53] carried out a study in which biopsies of the peri-implant mucosa were done in 20 patients 12 weeks following implant placement. The authors observed that, in patients without clinical signs of inflammation, the peri-implant mucosa had a mild inflammatory infiltrate. Berglundh and Donati [54] also described the presence of a chronic inflammatory infiltrate, dominated by plasma cells and B lymphocytes, in patients without clinical signs of inflammation.
Titanium has been considered an inert material, but recently, cases of type I or IV allergic reactions have been described, acting as irritant factors triggering the inflammatory process, leading to implant failure [55,56]. Sicilia et al. [57] performed a study on 1,500 patients with implants, observing positive allergic reactions in 0.6%.
In cases of dental implant mobility, at a microscopic level, metal particles can be released, potentially activating an inflammatory response, although its influence on carcinogenesis is unclear. Hydroxyapatite-coated implants aggravate this problem due to the different elasticity modulus between the coating and the underlying titanium [15]. Moxley et al. [29] presented a case in which two transmandibular posts were inserted. Two years following placement, they observed metal particles surrounding both posts, which had induced peri-implant mucositis and, a year after, one of the implants presented an OSCC in its vicinity.
Another factor that can trigger inflammation is corrosion, defined as “the deterioration of metal as a result of an electrochemical attack of the oral environment”. Most implants are made of titanium or titanium alloys, highly resistant to corrosion, due to the stability of the titanium dioxide (TiO2) layer that they present [15,55,58]. When this layer is deteriorated or removed, for example, following the mechanical debridement of bacterial plaque, by the presence of fluoride ions, by the friction of the implant against the supporting bone, or by an acidic environment within the oral cavity due to inflammation, titanium can suffer corrosion [58,59]. Corrosion also occurs between titanium implants and other metal alloys used in other dental procedures. Alloys composed of a less noble metal act as an anode, and the titanium of the implant as a cathode, transferring electrons by metallic contact, and releasing ions into the crevicular space, activating the host's immune response [55,59]. In addition, corrosion can also occur within the implant itself, because of cracks or microscopic holes along its surface acting as an anode, while the rest of the implant acts as a cathode. Some authors have associated the release of corrosive products with carcinogenesis and mutagenesis in the oral cavity [59]. In this regard, in 2006, the Agency for Research on Cancer considered TiO2 as a possible carcinogenic agent in humans [55,59]. Despite this, Doran et al. [60] concluded that titanium alloys are currently the safest for the manufacturing of dental implants, and that only vanadium, a minor component of their alloy, is toxic. In addition, the accumulation of a high concentration of metal ions in the oral cavity can act as an immunosuppressant agent locally, or they can be metabolized, generating cytotoxicity or potentially mutagenic reactive products [55,58].
OSCC recurrence
In many cases, regions of the jaws that have undergone resective surgery due to a previous OSCC are subsequently rehabilitated by implants which, along with other risk factors such as tobacco, alcohol, or the presence of potentially malignant disorders, could trigger the appearance of a new tumour. In this regard, locoregional recurrences have been described from 25 to 50% [61,62] and, orally, from 15 to 20% [24,39]. On the other hand, during radiotherapy, radiation can disperse, increasing dosage in front of the implant and decreasing it posteriorly. This theory, however, is difficult to associate with the recurrence of OSCC [27].
Pre-existence of potentially malignant disorders
In several of the described cases, the patients presented oral mucosal lesions, some of them initially benign, but following the insertion of a dental implant, became malignant. Either new lesions or recurrences may appear in the peri-implant mucosa in patients with a history of cancer or potentially malignant disorders [4,55].
In this regard, the pathogenesis of oral lichen planus (OLP) is not fully understood, but it is considered a potentially malignant disorder. According to Gonzáles-Moles et al. [63] the rate of malignant transformation of oral lichen planus in different studies carried out between 1924 and 2007 is established within a margin between 0% and 12.5%. It is considered a chronic inflammatory autoimmune disorder and, if we consider the effect of dental implants on the immune response and the development of chronic inflammation, it is reasonable to suggest that they could trigger the malignant transformation of this type of lesions. This malignant transformation is more likely to occur in atrophic and/or erosive lichen planus lesions [64,65]. In OLP, the response to chronic inflammation and the healing process can increase the number of genetic mutations [64]. This hypothesis seems to be confirmed by studies that illustrate the relationship between T-cell inflammatory mediators and carcinogenesis [66]. These studies show that the macrophage migration inhibitory factor (MIF), released by T-cells and macrophages, decreases p53 transcription, a protein considered as a tumour suppressant gene. Protein p53 plays an important role in carcinogenesis, eliminating mutant cells by apoptosis. In this regard, mutant p53 have been found in over 60% of cancers. P53 blockage by MIF (or other mediators) increases the risk of developing cancer in cases of OLP [67]. Despite this, further studies are needed to confirm this hypothesis [4,40].
Other disorders classified as potentially malignant are erythroplakia, erythroleukoplakia and leukoplakia [68]. Non-homogenous nodular or speckled leukoplakia present a higher risk of malignancy [65] along with verrucous proliferative leukoplakia, which is considered an early form of verrucous carcinoma [68]. Likewise, erythroplakia have a high rate of malignant transformation (14–50%). Another entity associated with a high rate of malignancy is submucosal oral fibrosis, which is estimated to be around 7–30%. Other malignancy risk factors to be considered in these patients are female gender, long-lasting lesions, presence of leukoplakia unrelated to smoking, lesion size greater than 200 mm2, non-homogeneous forms and presence of epithelial dysplasia [65].
Migration of malignant cells through the peri-implant groove
The potential route through which OSCC could spread is the invasion of the alveolar ridge, as well as the cortical bone [39,69]. Brown [69] studied other possible routes of dissemination, such as the periodontal ligament. However, as implants lack periodontal ligament, they do not fit into this theory. It is not known if the presence of dental implants could influence or modify the pattern of mandibular invasion. Most of the described cases emphasize that dental implants have not been clearly identified as a potential route of invasion of OSCC towards the jaws [39]. Only Schache et al. [24] suggest that the placement of dental implants could enable OSCC dissemination towards the alveolar bone, through the bone-implant interface. Therefore, further histological studies are needed to understand the possible dissemination routes, and their relationship, if any, with implants [5].
Metastatic lesions in soft peri-implant tissues
Some cases of metastasis in the vicinity of dental implants have been described [13,36,42–44]. It is important to consider that the pathogenesis of metastasis to the maxilla and mandible is not clear. One theory that could explain its pathogenesis is the vascular theory. The premolar and molar mandibular region represent a region rich in bone marrow. In this situation, blood flow decreases and causes a change in blood circulation, which could result in a greater trapping of metastatic cells [5,70]. Local factors, such as trauma or surgical procedures, could further increase this process, due to the trap of tumour cells during the formation of clots in recent wounds and to growth factors locally released by regenerated tissues, which would stimulate the formation and proliferation of malignant tumour cells.
Chronic inflammation also promotes metastasis locally because circulating tumour cells can become trapped within the rich capillary network of tissues that exhibit chronic inflammation. Favia et al. [43] describe a case of breast cancer metastasis around dental implants, in a patient under treatment with intravenous bisphosphonates (4 mg of zoledronic acid per month, for 33 months), who developed a peri-implant medication- related osteonecrosis of the jaws (MRONJ), not linked to the insertion of it. Upon histopathological analysis, metastatic deposits were observed within the peri-implant necrotic bone. Orhan et al. [44] describe another case of metastasis in a 69-year-old patient treated for breast cancer 18 years earlier undergoing annual check-ups, with no signs of disease recurrence. Two implants were placed in the mandibular premolar area, and 3 months later, the patient developed numb chin syndrome, along with chest pain, arthralgia, back pain, vomiting and perspiration that, once studied, confirmed a recurrence of breast cancer, with metastasis in different locations, among them, around the implants.
It is not possible to establish a link between dental implants and cancerous lesions. However, before considering implant placement, a careful assessment of the patient's medical history and potential risk factors is essential, particularly a possible history of cancer.
Conclusions
Implant treatment is not to be contraindicated in patients at risk, but any risk factors present at the time must be comprehensively controlled, as well as implementing a stricter recall protocol. Upon the appearance of lesions compatible with periimplantitis, a differential diagnosis should be made with OSCC and, in cases of the sudden appearance of hyperplastic or osteolytic lesions, unresponsive to conventional treatment and/ or anaesthesia or paraesthesia, a biopsy is crucial.
Due to the increasing popularity of dental implants, it is essential that, in turn, dentists who review these patients in the future are aware of the possible complications that may arise, among which the presence of malignant lesions could be found.
Conflicts of interests
The authors declare that they have no conflicts of interest in relation to the publication of this article.
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. [CrossRef] [PubMed] [Google Scholar]
- Attar E, Dey S, Hablas A, Seifeldin IA, Ramadan M, Rozek LS, et al. Head and neck cancer in a developing country: a population-based perspective across 8 years. Oral Oncol 2010;46: 591–596. [CrossRef] [PubMed] [Google Scholar]
- Bagan J, Sarrion G, Jimenez Y. Oral cancer: clinical features. Oral Oncol 2010;46:414–417. [CrossRef] [PubMed] [Google Scholar]
- Agha-Hosseini F, Rohani B. Evaluation of the effects of dental implants on oral lesions. J Contemp Dent Pract 2015;16: 400–406. [CrossRef] [PubMed] [Google Scholar]
- Pfammatter C, Lindenmuller IH, Lugli A, Filippi A, Kuhl S. Metastases and primary tumors around dental implants: A literature review and case report of peri-implant pulmonary metastasis. Quintessence Int 2012;43:563–570. [PubMed] [Google Scholar]
- Moergel M, Karbach J, Kunkel M, Wagner W. Oral squamous cell carcinoma in the vicinity of dental implants. Clin Oral Investig 2014;18:277–284. [CrossRef] [PubMed] [Google Scholar]
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 2009;45:309–316. [CrossRef] [PubMed] [Google Scholar]
- Izumo T, Kirita T, Ariji E, Ozeki S, Okada N, Okabe S, et al. General rules for clinical and pathological studies on oral cancer: a synopsis. Jpn J Clin Oncol 2012;42:1099–1109. [CrossRef] [PubMed] [Google Scholar]
- Noguchi M, Tsuno H, Ishizaka R, Fujiwara K, Imaue S, Tomihara K, et al. Primary peri-implant oral intra-epithelial neoplasia/carcinoma in situ: a case report considering risk factors for carcinogenesis. Int J Implant Dent 2017;3:47. [CrossRef] [PubMed] [Google Scholar]
- Lewin F, Norell SE, Johansson H, Gustavsson P, Wennerberg J, Biorklund A, et al. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: a population-based case-referent study in Sweden. Cancer 1998;82:1367–1375. [CrossRef] [PubMed] [Google Scholar]
- Lodi G, Scully C, Carrozzo M, Griffiths M, Sugerman PB, Thongprasom K. Current controversies in oral lichen planus: report of an international consensus meeting. Part 2. Clinical management and malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;100:164–178. [CrossRef] [PubMed] [Google Scholar]
- de Vries N, Van der Waal I, Snow GB. Multiple primary tumours in oral cancer. Int J Oral Maxillofac Surg 1986;15:85–87. [CrossRef] [PubMed] [Google Scholar]
- Dib LL, Soares AL, Sandoval RL, Nannmark U. Breast metastasis around dental implants: a case report. Clin Implant Dent Relat Res 2007;9:112–115. [CrossRef] [PubMed] [Google Scholar]
- Raiser V, Abu-El Naaj I, Shlomi B, Fliss DM, Kaplan I. Primary oral malignancy imitating Peri-Implantitis. J Oral Maxillofac Surg 2016;74:1383–1390. [CrossRef] [PubMed] [Google Scholar]
- Bhatavadekar NB. Squamous cell carcinoma in association with dental implants: an assessment of previously hypothesized carcinogenic mechanisms and a case report. J Oral Implantol 2012;38:792–798. [Google Scholar]
- Meijer GJ, Dieleman FJ, Berge SJ, Merkx MAW. Removal of an oral squamous cell carcinoma including parts of osseointegrated implants in the marginal mandibulectomy. A case report. Oral Maxillofac Surg 2010;14:253–256. [Google Scholar]
- De Ceulaer J, Magremanne M, van Veen A, Scheerlinck J. Squamous cell carcinoma recurrence around dental implants. J Oral Maxillofac Surg 2010;68:2507–2512. [CrossRef] [PubMed] [Google Scholar]
- Gulati A, Puthussery FJ, Downie IP, Flood TR. Squamous cell carcinoma presenting as peri-implantitis: a case report. Ann R Coll Surg Engl 2009;91:2507–2512. [Google Scholar]
- Gallego L, Junquera L, Llorente S. Oral carcinoma associated with implant-supported overdenture trauma: a case report. Dent Traumatol 2009;25:e3–e4. [CrossRef] [PubMed] [Google Scholar]
- Gallego L, Junquera L, Baladron J, Villarreal P. Oral squamous cell carcinoma associated with symphyseal dental implants: an unusual case report. J Am Dent Assoc 2008;139:1061–1065. [CrossRef] [PubMed] [Google Scholar]
- Kwok J, Eyeson J, Thompson I, McGurk M. Dental implants and squamous cell carcinoma in the at risk patient-report of three cases. Br Dent J 2008;205:543–545. [CrossRef] [PubMed] [Google Scholar]
- Eguia del Valle A, Martinez-Conde Llamosas R, Lopez Vicente J, Uribarri Etxebarria A, Aguirre Urizar JM. Primary oral squamous cell carcinoma arising around dental osseointegrated implants mimicking peri-implantitis. Med Oral Patol Oral Cir Bucal 2008;13:E489–E491. [Google Scholar]
- Chimenos-Kustner E, López-López J, Finestres-Zubeldia F. Squamous carcinoma after dental implants: a clinical case. Rev Port Estomatol Med Dentária e Cir Maxilofac 2008;49: 97–100. [Google Scholar]
- Schache A, Thavaraj S, Kalavrezos N. Osseointegrated implants: a potential route of entry for squamous cell carcinoma of the mandible. Br J Oral Maxillofac Surg 2008;46:397–399. [CrossRef] [PubMed] [Google Scholar]
- Abu El-Naaj I, Trost O, Tagger-Green N, Trouilloud P, Robe N, Malka G, et al. Peri-implantitis or squamous cell carcinoma? Rev Stomatol Chir Maxillofac 2007;108:458–460. [CrossRef] [PubMed] [Google Scholar]
- Czerninski R, Kaplan I, Almoznino G, Maly A, Regev E. Oral squamous cell carcinoma around dental implants. Quintessence Int 2006;37:707–711. [PubMed] [Google Scholar]
- Shaw R, Sutton D, Brown J, Cawood J. Further malignancy in field change adjacent to osseointegrated implants. Int J Oral Maxillofac Surg 2004;33:353–355. [CrossRef] [PubMed] [Google Scholar]
- Block MS, Scheufler E. Squamous cell carcinoma appearing as peri-implant bone loss: a case report. J Oral Maxillofac Surg 2001;59:1349–1352. [CrossRef] [PubMed] [Google Scholar]
- Moxley JE, Stoelinga PJ, Blijdorp PA. Squamous cell carcinoma associated with a mandibular staple implant. J Oral Maxillofac Surg 1997;55:1020–1022. [CrossRef] [PubMed] [Google Scholar]
- Clapp C, Wheeler JC, Martof AB, Levine PA. Oral squamous cell carcinoma in association with dental osseointegrated implants. An unusual occurrence. Arch Otolaryngol Head Neck Surg 1996;122:1402–1403. [CrossRef] [PubMed] [Google Scholar]
- Friedman KE, Vernon SE. Squamous cell carcinoma developing in conjunction with a mandibular staple bone plate. J Oral Maxillofac Surg 1983;41:265–266. [CrossRef] [PubMed] [Google Scholar]
- Brabyn P, Naval L, Zylberberg I, Muñoz-Guerra MF. Oral squamous cell carcinoma after dental implant treatment. Rev Esp Cir Oral y Maxilofac 2018;40:176–186. [CrossRef] [Google Scholar]
- Bhandari S, Rattan V, Panda N, Vaiphei K, Mittal BR. Oral cancer or periimplantitis: A clinical dilemma. J Prosthet Dent 2016; 115:658–661. [CrossRef] [PubMed] [Google Scholar]
- Malthiery E, De Boutray M, Koren C, Albouy J-P, Torres J-H, Fauroux M-A. Squamous cell carcinoma around a dental implant: a case report and literature review. Oral Oncol 2019. https://doi.org/10.1016/j.oraloncology.2019.02.005. [Google Scholar]
- Granados F, Santos-Ruiz L, Contreras M, Mellado J, Martin G, Bermudo L, et al. Squamous cell carcinoma related with dental implants. A clinical cases report. J Clin Exp Dent 2020;12: e98–e102. [CrossRef] [PubMed] [Google Scholar]
- Kaplan I, Zeevi I, Tal H, Rosenfeld E, Chaushu G. Clinicopathologic evaluation of malignancy adjacent to dental implants. Oral Surg Oral Med Oral Pathol Oral Radiol 2017;123: 103–112. [Google Scholar]
- Carreira-Nestares B, de Cáceres MA, Encinas-Bascones A, de Pedro M, Berguer-Sandez A. Carcinoma epidermoide oral alrededor de implantes osteointegrados: a propósito de un caso y revisión bibliográfica. Rev Chil Cirugía 2017. [Google Scholar]
- Chainani-Wu N, Chang C, Sim C, Wu T, Cox D, Sirjani D, et al. Oral squamous cell carcinoma mimicking Peri-Implantitis. Clin Adv Periodontics 2016;6:83–88. [PubMed] [Google Scholar]
- Nariai Y, Kanno T, Sekine J. Histopathological features of secondary squamous cell carcinoma around a dental implant in the mandible after chemoradiotherapy: a case report with a clinicopathological review. J Oral Maxillofac Surg 2016;74: 982–990. [CrossRef] [PubMed] [Google Scholar]
- Marini E, Spink MJ, Messina AM. Peri-implant primary squamous cell carcinoma: a case report with 5 years' follow-up. J Oral Maxillofac Surg 2013;71:322–326. [CrossRef] [PubMed] [Google Scholar]
- Moshref M, Jamilian A, Lofti A, Showkatbakhsh. Oral squamous cell carcinoma associated with dental implant − a case report and literature review. J Clin Exp Dent 2011;3:e166–e168. [Google Scholar]
- Verhoeven JW, Cune MS, van Es RJJ. An unusual case of implant failure. Int J Prosthodont 2007;20:51–54. [PubMed] [Google Scholar]
- Favia G, Tempesta A, Limongelli L, Crincoli V, Piattelli A, Maiorano E. Metastatic breast cancer in medication-related osteonecrosis around mandibular implants. Am J Case Rep 2015;16: 621–626. [CrossRef] [PubMed] [Google Scholar]
- Orhan K, Bayndr H, Aksoy S, Seker BK, Berberoglu A, Ozan O. Numb chin syndrome as a manifestation of possible breast cancer metastasis around dental implants. J Craniofac Surg 2011;22:942–945. [CrossRef] [PubMed] [Google Scholar]
- García-Cañas P, García-Cañas R, García-Rebollar R, Marín-García F. Carcinoma verrucoso periimplantario. A propósito de un caso. Sanid Mil 2013;69:38–42. [CrossRef] [Google Scholar]
- Norton MR. Dental implants: Potential relationship with cancer. Br Dent J 2017;222:224. [CrossRef] [PubMed] [Google Scholar]
- Ito K, Takahashi K, Eda T, Kondoh T, Goss A. Peri-implant squamous cell carcinoma. Aust Dent J 2017. https://doi.org/10.1111/adj.12581. [Google Scholar]
- Salgado-Peralvo AO, Arriba-Fuente L, Mateos-Moreno MV, Salgado-Garcia A. Is there an association between dental implants and squamous cell carcinoma? Br Dent J 2016;221:645–649. [CrossRef] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–545. [CrossRef] [PubMed] [Google Scholar]
- DE Souza MB, Curioni OA, Kanda JL, DE Carvalho MB. Serum and salivary macrophage migration inhibitory factor in patients with oral squamous cell carcinoma. Oncol Lett 2014;8: 2267–2275. [PubMed] [Google Scholar]
- Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci 2011;7:651–658. [CrossRef] [PubMed] [Google Scholar]
- Sarode GS, Sarode SC, Patil A, Anand R, Patil SG, Rao RS, et al. Inflammation and oral cancer: an update review on targeted therapies. J Contemp Dent Pract 2015;16:595–602. [CrossRef] [PubMed] [Google Scholar]
- Silva E, Felix S, Rodriguez-Archilla A, Oliveira P, Martins dos Santos J. Revisiting peri-implant soft tissue − histopathological study of the peri-implant soft tissue. Int J Clin Exp Pathol 2014;7:611–618. [PubMed] [Google Scholar]
- Berglundh T, Donati M. Aspects of adaptive host response in periodontitis. J Clin Periodontol 2005;32:87–107. [CrossRef] [PubMed] [Google Scholar]
- Camacho-Alonso F, Sánchez-Siles M, Gilbel-del Águila O. No evidence of genotoxic damage in a group of patients with titanium dental implants and different metal restorations in the oral cavity. Clin Implant Dent Relat Res 2015;17: 811–821. [CrossRef] [PubMed] [Google Scholar]
- Evrard L, Waroquier D, Parent D. Allergies to dental metals. Titanium: a new allergen. Rev Med Brux 2010;31:44–49. [PubMed] [Google Scholar]
- Sicilia A, Cuesta S, Coma G, Arregui I, Guisasola C, Ruiz E, et al. Titanium allergy in dental implant patients: a clinical study on1500 consecutive patients. Clin Oral Implants Res 2008; 19:823–835. [CrossRef] [PubMed] [Google Scholar]
- Özcan M, Hämmerle C. Titanium as a reconstruction and implant material in dentistry: advantages and pitfalls. Materials (Basel) 2012;5:1528–1545. [Google Scholar]
- Chaturvedi TP. An overview of the corrosion aspect of dental implants (titanium and its alloys). Indian J Dent Res 2009;20: 91–98. [CrossRef] [PubMed] [Google Scholar]
- Doran A, Law FC, Allen MJ, Rushton N. Neoplastic transformation of cells by soluble but not particulate forms of metals used in orthopaedic implants. Biomaterials 1998;19:751–759. [CrossRef] [PubMed] [Google Scholar]
- Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol 2010;21:252–261. [Google Scholar]
- Kearney PL, Watkins JM, Shirai K, Wahlquist AE, Fortney JA, Garrett-Mayer E, et al. Salvage resection for isolated local and/or regional failure of head/neck cancer following definitive concurrent chemoradiotherapy case series and review of the literature. McGill J Med 2011;13:29. [PubMed] [Google Scholar]
- Gonzalez-Moles MA, Scully C, Gil-Montoya JA. Oral lichen planus: controversies surrounding malignant transformation. Oral Dis 2008;14:229–243. [CrossRef] [PubMed] [Google Scholar]
- Lysitsa S, Najm SA, Lombardi T, Samson J. Oral lichen planus: natural history and malignant transformation. Med Buccale Chir Buccale 2007;13:19–29. [CrossRef] [Google Scholar]
- Yardimci G, Kutlubay Z, Engin B, Tuzun Y. Precancerous lesions of oral mucosa. World J Clin Cases 2014;16:866–872. [Google Scholar]
- Whyte DA, Broton CE, Shillitoe EJ. The unexplained survival of cells in oral cancer: what is the role of p53? J Oral Pathol Med 2002;31:125–133. [CrossRef] [PubMed] [Google Scholar]
- van der Meij EH, Schepman KP, Smeele LE, van der Wal JE, Bezemer PD, van der Waal I. A review of the recent literature regarding malignant transformation of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:307–310. [CrossRef] [PubMed] [Google Scholar]
- Alkan A, Bulut E, Gunhan O, Ozden B. Oral verrucous carcinoma: a study of 12 cases. Eur J Dent 2010;4:202–207. [PubMed] [Google Scholar]
- Brown J. Mechanisms of cancer invasion of the mandible. Curr Opin Otolaryngol Head Neck Surg 2003;11:96–102. [CrossRef] [PubMed] [Google Scholar]
- Shankar S. Dental pulp metastases and pan-osseous mandibular involvement with mammary adenocarcinoma. Br J Oral Maxillofac Surg 1984;22:455–461. [CrossRef] [PubMed] [Google Scholar]
All Tables
Summary of the cases described in the literature of metastasis, OSCC or clinical variants in the peri-implant mucosa (F, female; M, male; mand, mandible; max, maxilla; BCC, basal cell carcinoma; UNS, unspecified by the authors; OD, overdenture; FP, fixed prosthesis; OSCC, oral squamous cell carcinoma; AP, anatomopathological; VC, verrucous carcinoma; OH, oral hygiene; Tx, treatment; OLP, oral lichen planus).
All Figures
 |
Fig. 1 PRISMA® flow diagram of the search processes and results. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


